Henan Lantian Medical Supplies Co.,Ltd. |
|
Prior to testing, all reagents were returned to room
temperature.Testing shall be performed at room temperature.
I. Sample extraction (see Figure 1)
1. Add 400 L (about 10 drops) of sample buffer vertically to the
sample extraction tube and then insert the sample into the tube and
rotate against the inner wall about 10 times so that the samples in
the solution dissolve as much as possible.
2. Squsample q-tip along the inner wall of the extraction tube to
retain as much liquid in the tube as possible from the sample, then
removed and discarded.
3. Cover the dropper.

two.Test procedure (see Figure 2)
1. Remove the test card from the sealing bag.
2. Add 2 drops (about 80 L) of the treated sample extract to the
sample hole of the test card and start the timer.
3. The results were read after placing the test card for 15 minutes
at room temperature.The results were invalid after 20 minutes.

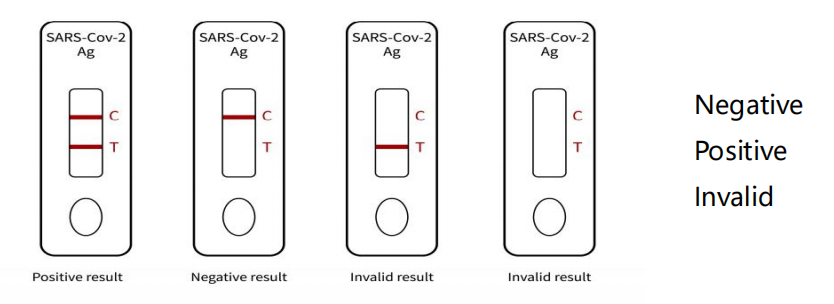
Schematic diagram of the test card result judgment:
Invalid ① result: the quality control line (C line) has no reaction
line, the detection is invalid, and the test shall be repeated.
② negative result: red ribbon, quality control line (C line) is in
color.
③ positive result: two red bands, detection line (T line) and
quality control line (C line) are in color.




