MAGNUS INTERNATIONAL LIMITED |
|
››› Reliable & Rapid Antigen Testing Kit!
››› Results in 15 minutes!!
››› Easy, Safe, Money-saving!!!
【PRODUCT NAME】
SARS-CoV-2 (COVID-19) Antigen Rapid Test Kit (Colloidal Gold)
【PACKAGE SPECIFICATION】
25 tests/kit & 50 tests/kit.
【INTENDED USE】
This kit is intended to be used for the qualitative detection of SARS-CoV-2 antigen in human
respiratory specimens, sputum, and other samples. This kit uses a cellulose membrane
immunochromatography technology.
Antigen detection is used for auxiliary diagnosis or epidemiological investigation of human
infection with SARS-CoV-2.
【COVID-19 RAPID ANTIGEN TEST】
The COVID-19 rapid antigen test detects protein fragments specific
to the
Coronavirus. Rapid antigen testing is a faster way to detect if you have a current
COVID-19 infection. Rapid antigen testing may be helpful for those who want
quick results to meet a testing requirement for an event or travel. For example,
this test can be used if you plan to visit a location or establishment that requires
testing prior to arrival. Testing requirements vary by location and may change
over time. This test requires a nasal or nasopharyngeal swab. With our product
testing, results may come back in as soon as 10 – 15 minutes.
Common COVID-19 symptoms include fever, cough, and shortness of breath.

An antigen test reveals if a person is currently infected with a pathogen such as
the SARS-CoV-2 virus. Once the infection has gone, the antigen disappears.
Unlike nucleic acid based tests such as PCR, which detect the presence of genetic
material, antigen tests detect proteins or glycans, such as the spike proteins found
on the surface of the SARS-CoV-2 virus.
They can take longer to develop than molecular and antibody tests, as suitable
antibodies for use in the assays must first be identified and produced, which can
be a complex and time-consuming process. Accuracy can also be a problem, with
antigen tests typically having a much lower sensitivity than PCR.
However, they usually provide test results rapidly, are relatively cheap, and can be
more amenable to point-of-care use, which could make them more suitable for
testing in the community and in remote regions.
All COVID-19 tests start with a sample, but the scientific process goes very differently
after that.
At this point in the pandemic, you or someone you know has probably received
at least one COVID-19 test. But do you know which kind of test you got and the
strengths and weaknesses of these different tests?
Two major types of tests are used to diagnose infection with SARS-CoV-2:
molecular tests – better known as PCR tests – and antigen tests. Each detects a
different part of the virus, and how it works influences the test’s speed and relative
accuracy. So, what are the differences between these types of tests?
PCR tests are extremely accurate but require special lab equipment – like the PCR
heating machine seen here – and can take hours or days to perform.
【Rapid Antigen tests】
Rapid, accurate tests are essential to contain a highly contagious virus like SARS-CoV-2.
PCR tests are accurate but can take a long time to produce results. Antigen tests,
the other major type of coronavirus test, while much faster, are less accurate.
Antigens are substances that cause the body to produce an immune response –
they trigger the generation of antibodies. These tests use lab-made antibodies to
search for antigens from the SARS-CoV-2 virus.
To run an antigen test, you first treat a sample with a liquid containing salt and soap
that breaks apart cells and other particles. Then you apply this liquid to a test strip
that has antibodies specific to SARS-CoV-2 painted on it in a thin line.
Just like antibodies in your body, the ones on the test strip will bind to any antigen
in the sample. If the antibodies bind to coronavirus antigens, a colored line appears
on the test strip indicating the presence of SARS-CoV-2.
Antigen tests have a number of strengths. First, they are so easy to use that people
with no special training can perform them and interpret the results – even at home.
They also produce results quickly, typically in less than 15 minutes. Another benefit
is that these tests can be relatively inexpensive at around $10-$15 per test.
Antigen tests do have some drawbacks. Depending on the situation, they can be
less accurate than PCR tests. When a person is symptomatic or has a lot of virus in
their system, antigen tests are very accurate. However, unlike molecular PCR tests,
antigen tests don’t amplify the thing they are looking for. This means there needs to
be enough viral antigen in the sample for the antibodies on the test strip to generate
a signal. When a person is in the early stages of infection, not a lot of virus is in the
nose and throat, from which the samples are taken. So, antigen tests can miss early
cases of COVID-19. It’s also during this stage that a person has no symptoms, so
they are more likely to be unaware they’re infected.
Understanding the strengths and limitations of both PCR and antigen tests, and
when to use them, can help to bring the COVID-19 pandemic under control.
So, the next time you get a COVID-19 test, choose the one that is right for you.
【How the test works?】
A sample is collected through a nasal or throat swab and mixed with
a solution. The solution is then placed onto an indicator device that may
detect the presence of the virus that causes COVID-19.
Test Purpose: Used to detect an active infection.
Sample Type: Nasal or Nasopharyngeal Swab
Results Time: 10 – 15 Minutes*
(Wait times may vary depending on volume)
All COVID-19 tests start with a sample, but the scientific process goes very
differently after that.
At this point in the pandemic, you or someone you know has probably received
at least one COVID-19 test. But do you know which kind of test you got and the
strengths and weaknesses of these different tests?
Two major types of tests are used to diagnose infection with SARS-CoV-2:
molecular tests – better known as PCR tests – and antigen tests. Each detects
a different part of the virus, and how it works influences the test’s speed and
relative accuracy. So, what are the differences between these types of tests?
PCR tests are extremely accurate but require special lab equipment – like the PCR
heating machine seen here – and can take hours or days to perform.
Rapid antigen tests offer a quick and easy way to screen for COVID-19 at home.
In about 15 minutes, they detect active infections via a nasal swab, including in
asymptomatic individuals. Often called “rapid tests” or “at-home COVID tests,”
these rapid antigen tests can be a valuable tool for managing life during the pandemic
—if you can get your hands on one.
Rapid antigen tests are used as a test to diagnose COVID-19.
This means that if you test positive using a rapid antigen test, your result
does not need to be confirmed with a PCR test.
In only 15 minutes, you will have the results you need to confidently return to work,
school, sports and all the things you love to do, with the ability to store and share
our family's and your test results from Renji Antigen Rapid Test.
With Renji COVID-19 Antigen Rapid Test, you can test yourself after being out in
public. Early and regular testing helps you better care for yourself and protects
your friends, family, and community members from potential exposure.
【When should I use a rapid antigen test?】
If you are a close contact, you can use a rapid antigen test for your initial and day
6 test. You can also purchase and use rapid antigen tests from us:
or worried.
Accessing rapid antigen tests
If you are not a close contact, rapid antigen tests are sold
commercially in
supermarkets and pharmacies.
【KIT COMPONENTS】
| Components | 25T | 50T |
| 25 pcs | 50 pcs |
| 25 pcs | 50 pcs |
| 25 pcs | 50 pcs |
| 1 copy | 1 copy |
| Note: The components in the kits of different batch numbers are not interchangeable. | ||
The materials and instruments necessary for the test but not provided are as follows:
Lancet;
Absorbent paper or similar material;
Timer;
Micropipette corresponding to the range;
Laboratory safety protection equipment such as disposable gloves, etc.

【EXPERIMENT WORKFLOW】
Please read the instruction for use carefully before using this kit. All reagents should be
incubated at room temperature (10-30°C) for 30 minutes prior to use. The test should be
carried out at room temperature and the operation procedure is described below:
1. Open the sealed bag and remove the Detection Strip. Mark the sample ID on the test strip
and lay the strip flat on the table.
2. Specimen collection
1). Carefully insert the swab into the nostril of the patient, reaching the surface of the posterior
nasopharynx, that presents the most secretion under visual inspection.
2). Swab over the surface of the posterior nasopharynx. Rotate the swab several times.
3). Withdraw the swab from the nasal cavity.

3. Sample preparation
1). Insert the sample diluent tube into the pipe rack, make sure that the tube is standing firm
and reaches the bottom of the pipe rack.
2). Open the purple cap of the sample diluent tube. Insert the swab into the diluent tube which
contains 0.5 mL of the diluent buffer. Roll the swab at least 6 times while pressing the head
against the bottom and side of the diluent tube.
3). Leave the swab in the diluent tube for 1 minute.
4). Squeeze the tube several times with fingers from outside of the tube to immerse the swab.
Remove the swab, close the cap. The diluent solution will be used as test sample.
5). Open the small cap on the top of the sample dilution tube. Add 3-4 drops (~100 μL) of the
sample diluent buffer immediately to the sample well.
6). Allow the strip to develop for 10-15 minutes at room temperature. A visible band can be
read by naked eyes.

【RESULTS ANALYSIS】
The interpretation of visual interpretation results (as shown below):
1. The results can be read directly by naked eyes as shown in the figures below: Positive result:
a visible band can be seen in both line C and line T.

2. Negative result: A visible band can be seen in line C only.
3. Invalid result: A visible band cannot be seen in the line C, and the test must be repeated
using a new strip.
【MANAGEMENT OF RESULTS】

【REPORTING YOUR RESULT】
You are legally required to report all positive rapid antigen test
results. You must
report your positive result if you get your test from a designated RAT Collection
Point, from a retail outlet, from your workplace or through any other means
(i.e. over the internet).
You can also report negative and invalid test results to help
provide a full picture
of rates of COVID-19 testing in the state.
【WHAT DO I DO IF I HAVE A POSITIVE RESULT?】
【WHAT DO I DO IF I HAVE A NEGATIVE RESULT?】
If you are close contact with no COVID-19 symptoms and test negative, you do not
need to get a PCR test to confirm your result.
If you have COVID-19 symptoms and test negative, you must get a PCR test to
confirm your result. Rapid antigen tests are not as sensitive as PCR tests, which
means that just because you have a negative result in a rapid antigen test, it does
not mean that you do not have COVID-19.
【CUT-OFF VALUE】
1. Determination of the cut-off value, the product is a qualitative detection kit of antigens to
the SARS-CoV-2. When the quality control line (C line) and the test line (T line) are formed,
regardless of a weak T line, as long as it is visible to the normal naked eyes, the test result
should be judged as positive.
2. The test for the normal population should be negative. Positive test results indicate that an
individual may have been exposed to SARS-CoV-2, and should be combined with clinical
symptoms and other diagnostic results for further confirmation.
【INTERPRETATION OF TEST RESULTS】
1. Common mistakes that may lead to false-positive or negative results include: the kit is used
after its expiration; the kit has been improperly stored; the operating temperature is too low
(<4°C) or too high (>30°C); the procedures outlined in this protocol were not strictly adhered to.
2. Any final clinical interpretation should consider a combination of test results, clinical
symptoms, and other indicators.
【LIMITATIONS OF INSPECTION METHODS】
1. If the patient has clinical symptoms but the test result is negative, it is recommended to use
the PCR method for confirmation and the doctor will make a comprehensive judgment to
confirm the diagnosis. The negative result cannot be the only evidence to rule out the
SARS-CoV-2 infection.
2. The product can only be used for clinical diagnosis and on-site screening of the SARS-CoV-2.
The positive results of all Detection Strips must be confirmed by a qualified laboratory. The
positive result of antigen detection should be combined with other clinical features to ensure
an accurate diagnosis.
3. In order to ensure the accuracy of the SARS-CoV-2 antigens detection, high temperature
and humidity must be avoided.
【WARNINGS】
【STORAGE CONDITIONS AND SHELF LIFE】
Stored at 2°C ~ 30°C, and kept away from direct sunlight.
The validity period is 12 months.
After the aluminum foil bag is opened, the Detection Strip should be used within 1 hour.
The Sample Diluent Buffer should be capped immediately after usage.
Usage after the date of expiration is not recommended.
The date of manufacture & expiration is indicated on the label and package.
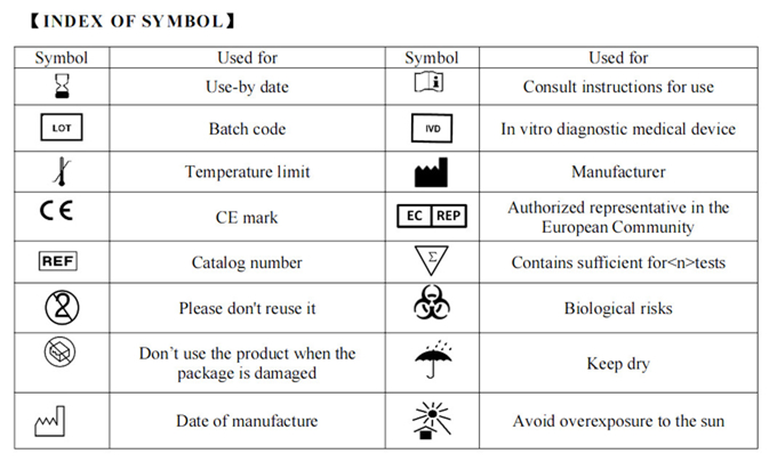
【INDEX OF SYMBOL】
【MANUFACTURER】
Changsha Renji Medical Equipment Co., Ltd.
No.18 Xiangtai Road, Liuyang Jingkai District,
Changsha City, Hunan Province 410300 China
【AUTHORIZED REPRESENTATIVE】
Lotus NL B.V.
Koningin Julianaplein 10, 1e Verd, 2595AA, The Hague, Netherlands.
【FACTORY VIEW】


【FACTORY WORKSHOP】

【PRODUCT AND STANDARDS】

【CERTIFICATIONS】


【FAQ】

