Labnovation Technologies, Inc. |
|
Rapid Antigen Test Kit SARS-CoV-2 Antigen Rapid Diagnostic Test Professional Testing CE Certificated 20Tests/Kit
Intend Use
The Labnovation SARS-CoV-2 antigen rapid test kit is intended for
the qualitative detection od SARS-COV-2 infection from
patients. It is used for in vitro qualitative detection of the antigen of novel
virus in human nasopharyngeal or Oropharyngeal swabs.
Product Details
| Item | Value |
| Model Number | LX-401301 |
| Type | 20 Tests/Kit |
| Warranty | 24 Months |
| Quality Certification | CE |
| Safty Standard | ISO13485 |
| Sample Type | Nasopharyngeal / Oropharyngeal Swab |
| Sample Volume | 3 Full drops |
Main Components

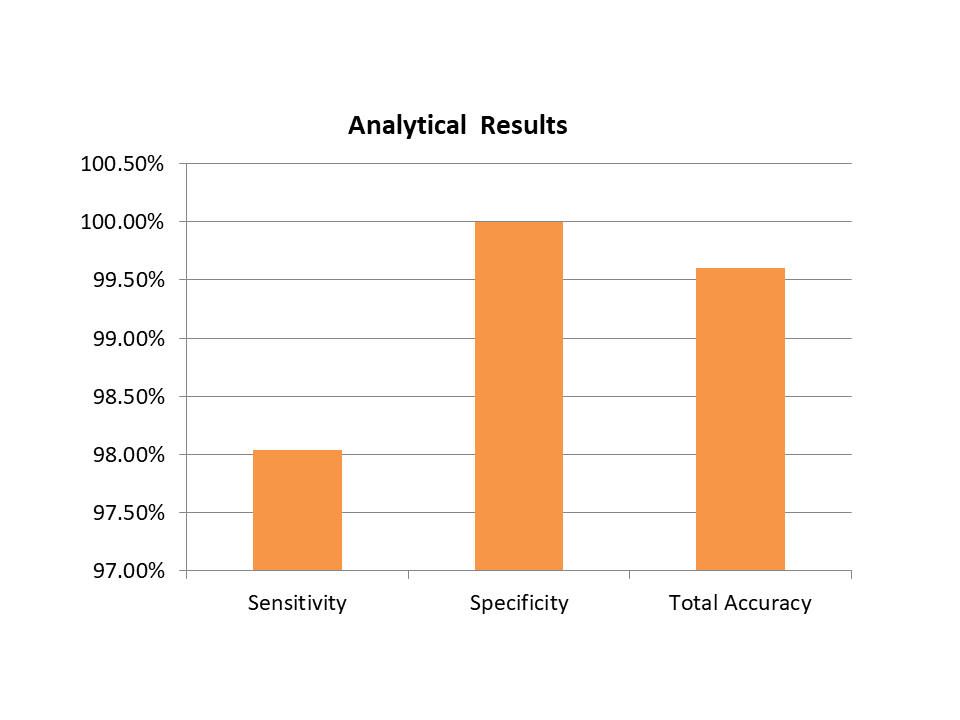
Analytical Results
| SARS-CoV-2 Antigen Rapid Test Kit | ||
| Sensitivity | Specificity | Total Accuracy |
| 98.04% | 100.00% | 99.60% |
Product Features

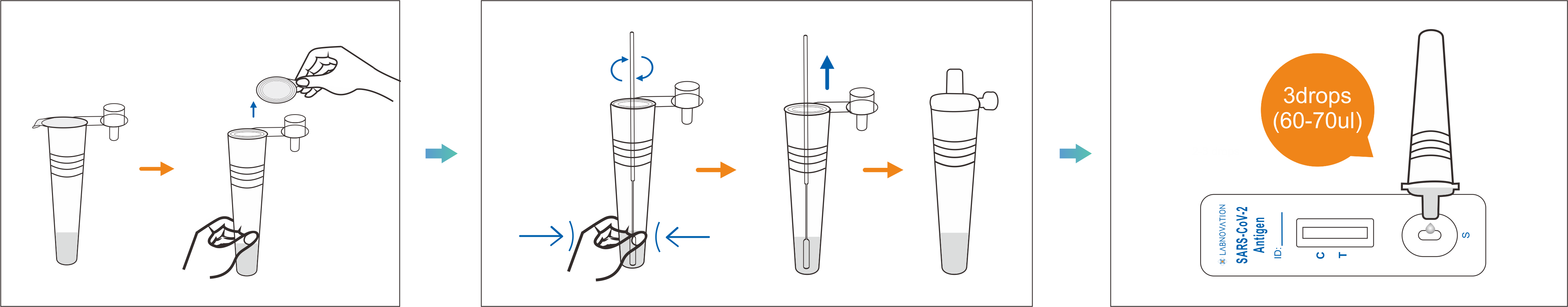
Sample Collection
Test steps
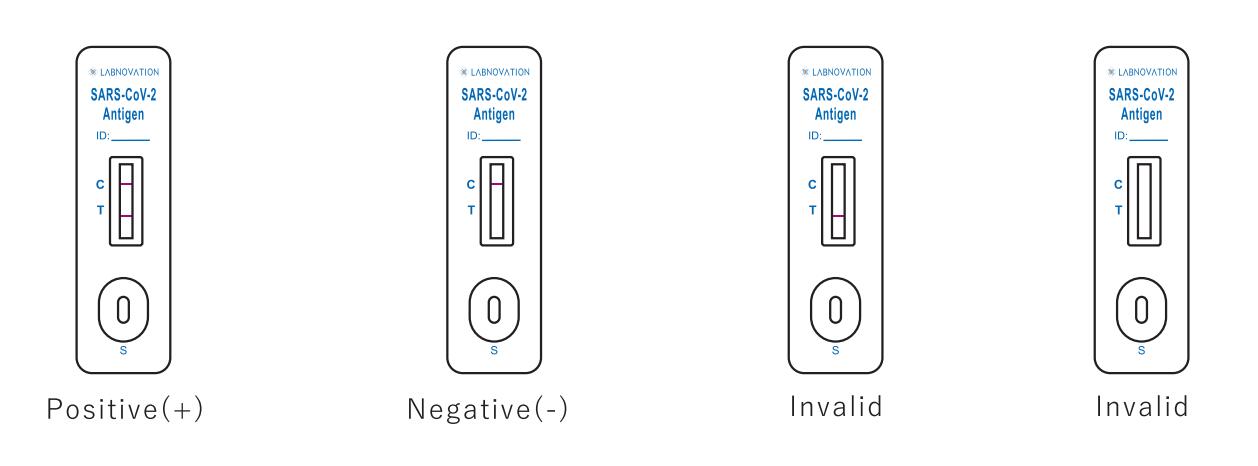
Result Interpretation
Positive: Two colored bands appear on the membrane. One band appears in the control region (C) and another band appears in the test region (T).
Negative: Only one colored band appears, in the control region (C). No apparent colored band appears in the test region (T).
Invalid: If there is no Control line (C) or only a Test line (T) in the result window, the test did not run correctly and the results are not valid.
Virus Sources
| Global high frequency mutation | Alpha / B.1.1.7(U.K.) | Beta I B.1.351(South Africa) |
| Gemma I P.1(Brazil) | Kappa I B.1.617.1(India) | Delta I B.1.617.2(India) |
| C.37,ect | Alpha I B.1.17(U.K.) | B.1.36.16.etc |
| A.2.5,etc | A.23.1 | Alpha I B.1.17(U.K.) |
| B.1.1.33.etc | C.1.1.etc. | Omicron |
Limitation
1. This reagent is a qualitative detection reagent, which cannot determine the exact content of antigen.
2. The test results of this reagent are only for the reference of clinicians and should not be taken as the sole basis for clinical diagnosis and treatment. Clinical management of patients should be considered in the light of their symptoms/signs, medical history, other laboratory tests and treatment responses.
3. Restricted by antigen detection reagent method, the lowest detection limit (sensitivity analysis) is generally lower than that of nucleic acid detection, so the researchers deal with negative result to give more attention, should be combined with other test results comprehensive judgment, advice to doubt the negative result of nucleic acid detection or virus isolation culture identification method for review.
4.False negative results may be caused by unreasonable sample collection, transport and treatment, and low viral load in samples.
Certificate