Dewei Medical Equipment Co., Ltd |
|
Verified Suppliers
|
|
【INTENDED USE】
The Strep A Rapid Test (Swab) is a rapid visual immunoassay for the qualitative, presumptive detection of Group A Streptococcus antigens in human throat swab specimens. This kit is intended for use as an aid in the diagnosis of Strep A infection.
【INTRODUCTION】
Beta-hemolytic Group A Streptococcus is a major cause of upper respiratory infections such as tonsillitis, pharyngitis, and scarlet fever. Early diagnosis and treatment of Group A Streptococcal pharyngitis has been shown to reduce the severity of symptoms and further complications, such as rheumatic fever and glomerulonephritis. Conventional methods for detecting Strep A infection are dependent on isolation and subsequent identification of the organism, and often require 24- 48 hours. Recent development of immunological techniques to detect Group A Streptococcal antigen directly from throat swabs allow physicians to diagnose and administer therapy immediately.
【PRINCIPLE】
The Strep A Rapid Test (Swab) detects Group A Streptococcus antigens through visual interpretation of color development on the internal strip. Anti-Strep A antibodies are immobilized on the test region of the membrane. During the test, the specimen reacts with polyclonal anti-Strep A antibodies conjugated to colored particles and precoated onto the sample pad of the test. The mixture then migrates through the membrane by capillary action and interacts with reagents on the membrane. If there is sufficient Strep A antigen in the specimen, a colored band will form at the test region of the membrane. The presence of this colored band indicates a positive result, while its absence indicates a negative result. The appearance of a colored band at the control region serves as a procedural control, indicating that proper volume of specimen has been added and membrane wicking has occurred.
【MAIN CONTENTS】
• Rapid test cassette with desiccant.
• Sampling Swab
• Reagent 1
• Reagent 2
• Extraction Tube
• Package insert
【PRECAUTIONS】
【STORAGE AND STABILITY】
• Store at 2 ~ 30 º C in the sealed pouch for 24 months.
• Keep away from direct sunlight, moisture and heat.
• DO NOT FREEZE.
【DIRECTION OF USE】
Bring tests, specimens, buffer and/or controls to room temperature (15- 30°C) before use.
1. Prepare swab specimens:
1) Place a clean extraction tube in the designated area of the workstation. Add 5 drops of Reagent 1 to the extraction tube, then add 4 drops of Reagent 2. Mix the solution by gently swirling the extraction tube.
1) Immediately immerse the swab into the extraction tube. Use a circular motion to roll the swab against the side of the extraction tube so that the liquid is expressed from the swab and can reabsorb.
2) Let stand for 1-2 minutes at room temperature, then squeeze the swab firmly against the tube to expel as much liquid as possible from the swab. Cap the extraction tube with the attached dropper tip. Discard the swab following guidelines for handling infectious agents.
2. Remove the test from its sealed pouch, and place it on a clean, level surface. Label the cassette with patient or control identification. For best results, the assay should be performed within one hour.
3. Add 3 drops (approximately 120 µL) of extracted solution from the extraction tube to the sample well on the test cassette. Avoid trapping air bubbles in the specimen well (S), and do not add any solution to the observation window. As the test begins to work, color will migrate across the membrane.
4. Wait for the colored band(s) to appear. The result should be read at 10 minutes. Do not interpret the result after 15 minutes.
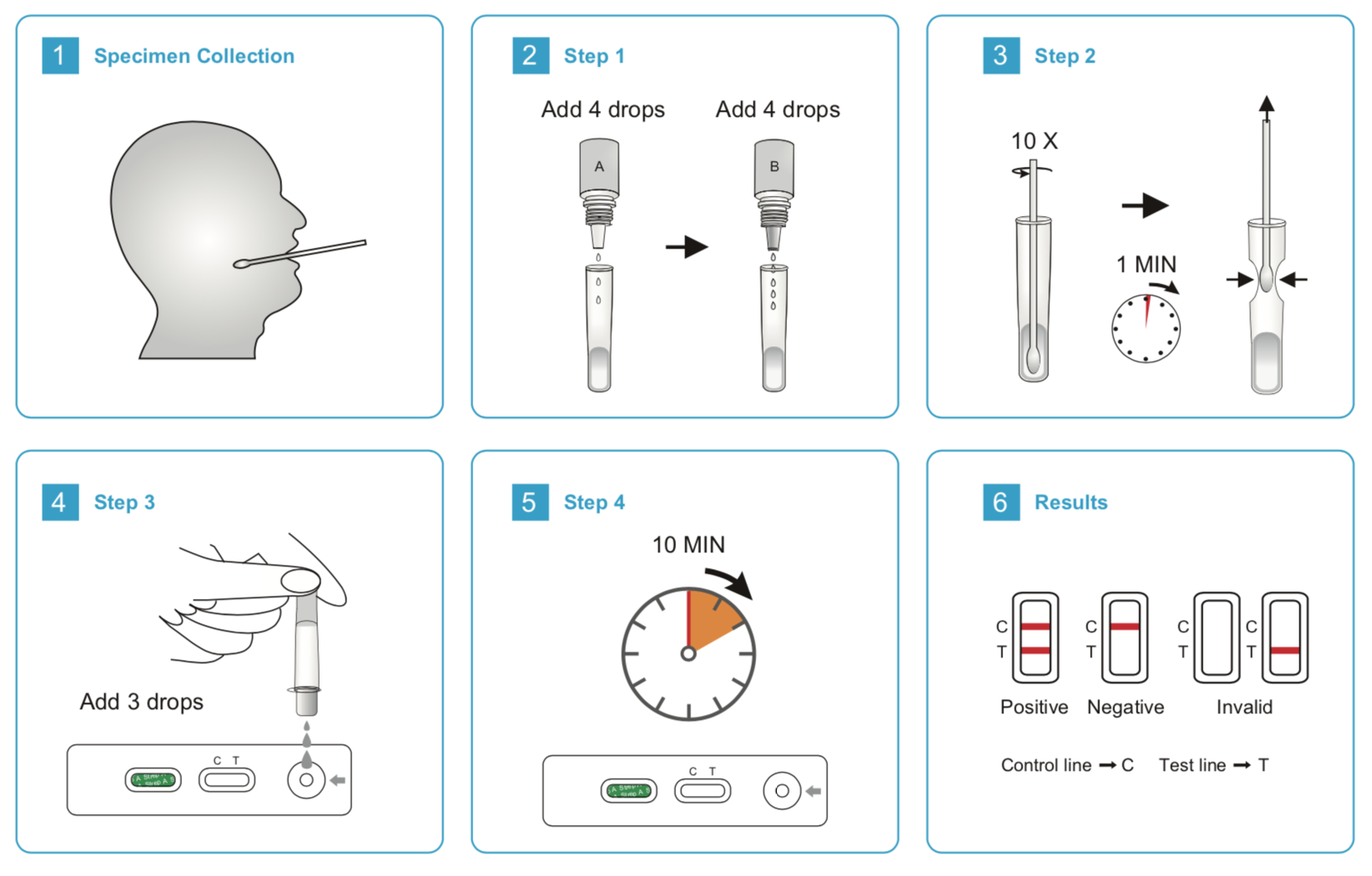
【INTERPRETATION OF RESULTS】
POSITIVE: The presence of two lines as control line (C) and test line (T) within the result window indicates a positive result.
NEGATIVE: The presence of only control line (C) within the result window indicates a negative result.
INVALID: If the control line (C) is not visible within the result window after performing the test, the result is considered invalid. Some causes of invalid results are because of not following the directions correctly or the test may have deteriorated beyond the expiration date. It is recommended that the specimen be re-tested using a new test.