SARS-CoV-2 Neutralization Antibody Rapid Test (Whole
Blood/Serum/Plasma), rapid chromatographic immunoassay
A rapid test for the qualitative detection of SARS-CoV-2
Neutralization Antibody in human whole blood, serum or plasma specimens.
For professional in vitro diagnostic use only.
| Product features | Parameters |
| Principle | Chromatographic Immunoassay |
| Format | Cassette |
| Specimen | WB/S/P |
| Certificate | CE |
| Reading Time | 10 minutes |
| Pack | 25T |
| Storage Temperature | 2-30°C |
| Shelf Life | 2 Years |
INTENDED USE
The SARS-CoV-2 Neutralization Antibody Rapid Test (Whole
Blood/Serum/Plasma) is a rapid chromatographic immunoassay intended for the qualitative
detection of neutralization antibodies against SARS-CoV-2 that block the
interaction between the receptor binding domain of the viral spike glycoprotein (RBD) with
the cell surface receptor ACE2 in human whole blood, serum or plasma. It is intended
for use as an aid in identifying individuals with an adaptive immune response to
SARS-CoV-2.
Results are for the detection of SARS-CoV-2 neutralization
antibodies. Positive results indicate the presence of neutralization antibodies to SARS-CoV-2.
SUMMARY
The novel coronaviruses belong to the β genus. COVID-19 is an acute
respiratory infectious disease. People are generally susceptible. Currently,
the patients infected by the novel coronavirus are the main source of infection;
asymptomatic infected people can also be an infectious source. Based on the current
epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main
manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose,
sore throat, myalgia and diarrhea are found in a few cases.
All coronaviruses share similarities in the organization and
expression of their genome, in which 16 nonstructural proteins (nsp1 through nsp16),
encoded by open reading frame (ORF) 1a/b at the 5’ end, are followed by the
structural proteins spike (S), envelope (E), membrane (M), and nucleocapsid
(N), which are encoded by other ORFs at the 3’ end.1 The virus gains entry to the
host cell through binding of the S protein receptor-binding domain (RBD) to the
angiotensin-converting enzyme 2 (ACE2) receptor on target cells, particularly respiratory
epithelial cells of the host. Upon infection with SARS-CoV-2, the host usually mounts an
immune response against the virus by producing different types of antibodies in the
blood.
A subset of these antibodies, which reduce viral infectivity by binding to the
surface epitopes of viral particles and thereby blocking the entry of the virus to an
infected cell, are defined as neutralizing antibodies (NAbs).
PRINCIPLE
This test contains two key components: the recombinant SARS-CoV-2
RBD fragment , labeled by colloidal gold, as tracers; and the human ACE2 receptor
protein (hACE2) , coated with cellulose nitrate membrane. When specimens are added to
the sample pad, neutralizing antibodies, if present in the specimen, will bind to
the RBD labeled colloidal gold and block the protein-protein interaction between RBD and
hACE2. The unbound RBD labeled colloidal gold as well as any RBD labeled colloidal
gold bound to non-neutralizing antibody will be captured on the test line. The
control line acts as a procedural quality control.
REAGENTS
The test contains recombinant SARS-CoV-2 RBD fragment coated
particles as a detection reagent and human ACE2 receptor protein coated with
cellulose nitrate membrane as a capture reagent.
PRECAUTIONS
1. This package insert must be read completely before performing
the test. Failure to follow directions in package insert may yield inaccurate test
results.
2. For professional in vitro diagnostic use only. Do not use after
the expiration date.
3. Do not eat, drink or smoke in the area where the specimens or
kits are handled.
4. Do not use the test if the pouch is damaged.
5. Handle all specimens as if they contain infectious agents.
Observe established precautions against microbiological hazards throughout the
collection, handling, storage and disposal of patient samples and the disposal of used
kit contents.
6. Wear protective clothing such as laboratory coats, disposable
gloves and eye protection when specimens are assayed.
7. Wash hands thoroughly after testing.
8. Please ensure that appropriate amounts of samples are used for
testing. Too much or too little may lead to deviation of results.
9. The used test should be discarded according to local
regulations.
10.Humidity and temperature can adversely affect results.
STORAGE AND STABILITY
Store as packaged in the sealed pouch at room temperature or
refrigerated (2-30°C). The test is stable until the expiration date printed on the sealed
pouch. The test must remain in the sealed pouch until use. DO NOT FREEZE. Do not use
beyond the expiration date.
DIRECTIONS FOR USE
Allow the test, specimen, buffer and/or controls to equilibrate to
room temperature (15-30°C) prior to testing.
1. Take out the test from the foil pouch and use it as soon as
possible. Best results will be obtained if the assay is performed within one hour.
2. Place the test on a flat and clean surface.
For Serum or Plasma specimen:
•Use a dropper: Hold the dropper vertically and transfer 2 drops of
serum or plasma
(approximately 50uL) to the specimen well (S). Start the timer.
•Use a pipette: Transfer 50uL of serum or plasma to the specimen
well(S), then
start the timer.
For Whole Blood specimen:
•Use a dropper: Hold the dropper vertically and transfer 3 drops of
whole blood
(approximately 75uL) to the specimen well (S). Then add 1 drop of
buffer
(approximately 40uL) and start the timer.
•Use a pipette: Transfer 75uL of whole blood to the specimen
well(S), then add 1
drop of buffer (approximately 40uL) and start the timer.
3. Wait for the colored line(s) to appear. Read results at 10
minutes. Do not interpret the result after 20 minutes.
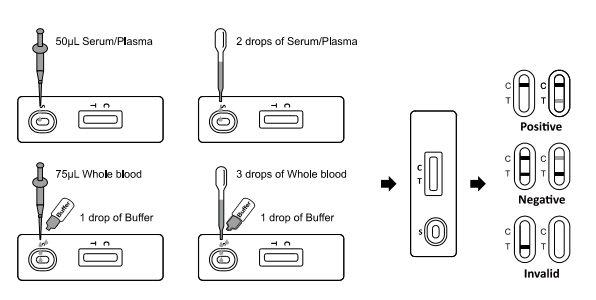
INTERPRETATION OF RESULTS
(Please refer to the illustration above)
POSITIVE: One colored line appears in the control region (C) or two
colored lines appear, color intensity of test region (T) is weaker
than line in control region (C). Positive result indicates the
detection of neutralization antibodies against SARS-CoV-2 in the
sample.
NEGATIVE: Two colored lines appear. One colored line should be in
the control region (C) and another colored line in the test region
(T). The color intensity of test region (T) is equal to or stronger
than control region (C).
Negative result indicates the neutralization antibody against
SARS-CoV-2 was not in the sample.
INVALID: Control line fails to appear. Insufficient specimen or
incorrect procedural techniques are the most likely reasons for
control line failure. Review the procedure and repeat the test with
a new test. If the problem persists, discontinue using the test kit
immediately and contact your local distributor.
LIMITATIONS
1. The test procedure and the interpretation of test result must be
followed closely when testing for the presence of neutralization
antibodies against SARS-CoV-2 in human whole blood, serum, or
plasma. For optimal test performance, proper sample collection is
critical. Failure to follow the procedure may give inaccurate
results.
2. The performance of the SARS-CoV-2 Neutralization Antibody Rapid
Test (Whole Blood/Serum/Plasma) was evaluated using the procedures
provided in this product insert only. Modifications to these
procedures may alter the performance of the test.
3. The SARS-CoV-2 Neutralization Antibody Rapid Test (Whole
Blood/Serum/Plasma) is for in vitro diagnostic use only. This test
should be used for detection of neutralization antibodies against
SARS-CoV-2 in human whole blood, serum, or plasma specimens.
Neither the quantitative value nor the rate of increase in the
concentration of neutralization antibodies against SARS-CoV-2 can
be determined by this qualitative test.
4. The hematocrit level of the whole blood can affect the test
results. Hematocrit level needs to be between 25% and 65% for
accurate results.
5. The test will show negative results under the following
conditions: The titer of the novel coronavirus antibodies in the
sample is lower than the minimum detection limit of the test, or
the neutralization antibody against SARS-CoV-2 has not appeared at
the time of sample collection.
6. Results from immunosuppressed patients should be interpreted
with caution.
Order Information
| Cat. No. | Product | Specimen | Pack |
| ICN-402 | SARS-CoV-2 Neutralization Antibody Rapid Test | WB/S/P | 25T |
