CITEST DIAGNOSTICS INC. |
|
Verified Suppliers
|
|
7-Aminoclonaze/pam (7-ACL) Dispoable Rapid Drug Abuse 300ng/mL* Test Kit OEM Diagnosis of CE
Applications:
The ACL Rapid Test Dipstick (Urine) is a rapid chromatographic immunoassay for the detection of 7-Aminoclonaze/pam (major metabolite) in urine at a cut-off concentration of 100ng/ml. This test will detect other related compounds, please refer to the Analytical Specificity table in this package insert.
This assay provides only a qualitative, preliminary analytical test
result. A more specific alternate chemical method must be used in
order to obtain a confirmed analytical result. Gas
chromatography/mass spectrometry (GC/MS) is the preferred
confirmatory method. Clinical consideration and professional
judgment should be applied to any drug of abuse test result,
particularly when preliminary positive results are used.
Description:
How to use?
Allow the test, urine specimen, and/or controls to reach room
temperature (15-30°C) prior to testing.
1. Bring the pouch to room temperature before opening it. Remove
the test cassette from the sealed pouch and use it within one hour.
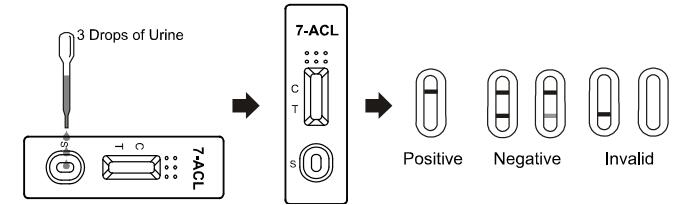
2. Place the test cassette on a clean and level surface. Hold the
dropper vertically and transfer 3 full drops of urine (approx.
120μL) to the specimen well (S) of the test cassette, and then
start the timer. Avoid trapping air bubbles in the specimen well
(S). See the illustration below.
3. Wait for the colored line(s) to appear. Read results at 5
minutes. Do not interpret the result after 10minutes.
DIRECTIONS FOR PANEL USE
Allow the test, urine specimen, and/or controls to reach room
temperature (15-30ºC) prior to testing.
1. Bring the pouch to room temperature before opening it. Remove
the test panel from the sealed pouch and use it within one hour.
2. Remove the cap.
3. With the arrow pointing toward the urine specimen, immerse the
test panel vertically in the urine specimen for at least 10 to 15
seconds. Immerse the strip to at least the level of the wavy lines,
but not above the arrow on the test panel.
4. Replace the cap and place the test panel on a non-absorbent flat
surface.
5. Start the timer and wait for the colored line(s) to appear.
6. The result should be read at 5 minutes. Do not interpret the
result after 10 minutes.

DIRECTIONS FOR DIPSTICK USE
Allow the test, urine specimen, and/or controls to reach room
temperature (15-30ºC) prior to testing.
1. Bring the pouch to room temperature before opening it. Remove
the test dipstick from the sealed pouch and use it within one hour.
2. With arrows pointing toward the urine specimen, immerse the test
dipstick vertically in the urine specimen for at least 10-15
seconds. Do not pass the maximum line (MAX) on the Test Dipstick
when immersing the strip. See the illustration below.
3. Place the test dipstick on a non-absorbent flat surface, start
the timer and wait for the colored line(s) to appear. Read results
at 5 minutes. Do not interpret the result after 10 minutes.

INTERPRETATION OF RESULTS
(Please refer to the illustration above)
NEGATIVE:* Two lines appear. One colored line should be in the
control line region (C), and another apparent colored line should
be in the test line region (T). This negative result indicates that
the 7-Aminoclonazepam (7-ACL) concentration is below the detectable
cut-off level.
*NOTE: The shade of color in the test line region (T) may vary, but
it should be considered negative whenever there is even a faint
colored line.
POSITIVE: One colored line appears in the control line region (C).
No line appears in the test line region (T). This positive result
indicates that the 7-Aminoclonazepam (7-ACL) concentration exceeds
the detectable cut-off level.
INVALID: Control line fails to appear. Insufficient specimen volume
or incorrect procedural techniques are the most likely reasons for
control line failure. Review the procedure and repeat the test with
a new test. If the problem persists, discontinue using the test kit
immediately and contact your local distributor.
| Cat. No. | Product Description | Specimen | Format | Kit Size | Cut-Off | Status |
| DACL-102 | 7-Aminoclonazepam (7-ACL) Rapid Test Cassette | Urine | Cassette | 40 T | 300ng/mL* | CE |
| DACL-114 | 7-Aminoclonazepam (7-ACL) Rapid Test Panel | Urine | Panel | 40 T | CE | |
| DACL-101 | 7-Aminoclonazepam (7-ACL) Rapid Test Dipstick | Urine | Dipstick | 50 T | CE |