Rapid Test Kit Neutralizing Antibody Rapid Test Kit High Quality
Detection Rapid Antibody Test Kit
Intended Use
- The Labnovation's SARS-CoV-2 neutralizing antibody rapid test kit
is intended for the semi-quantitative determination of neutralizing
antibodies to SARS-CoV-2 in human serum, plasma, fingersitick whole
blood or venous whole blood specimens.
- The test is intended use as an aid in identifying individuals with
an adaptive immune response to SARS-COV-2, indicating recent or
prior infection or assisting in evaluating the effectiveness of the
vaccine clinical trials and mass vaccination.
- Results are for the detection of total neutralizing antibodies to
SARS-COV-2. Antibodies to SARS-COV-2 are generally detectable in
blood several days after initial infection.
Specifications
| Test Item | Neutralizing Antibody Test Kit |
| Number | LX-401701 |
| Specificity | 98.00% |
| Sensitivity | 97.67% |
| Total Acuracy | > 95.00% |
| Humidity | 35%-60% |
| Storage Temperature | 2-30°C |
| Sample Type | Whole bloo, Serum, Plasma |
| Sample Volume | Whole blood 20µL, Serum/Plasma 10µL |
| Package | 20 Tests/Kit |
| nspection Method | Immunochromatography |
| Shelf Life | 18 Months |
| Type | IVD Antibody Rapid Test |
| Application | For Professional Testing Use |
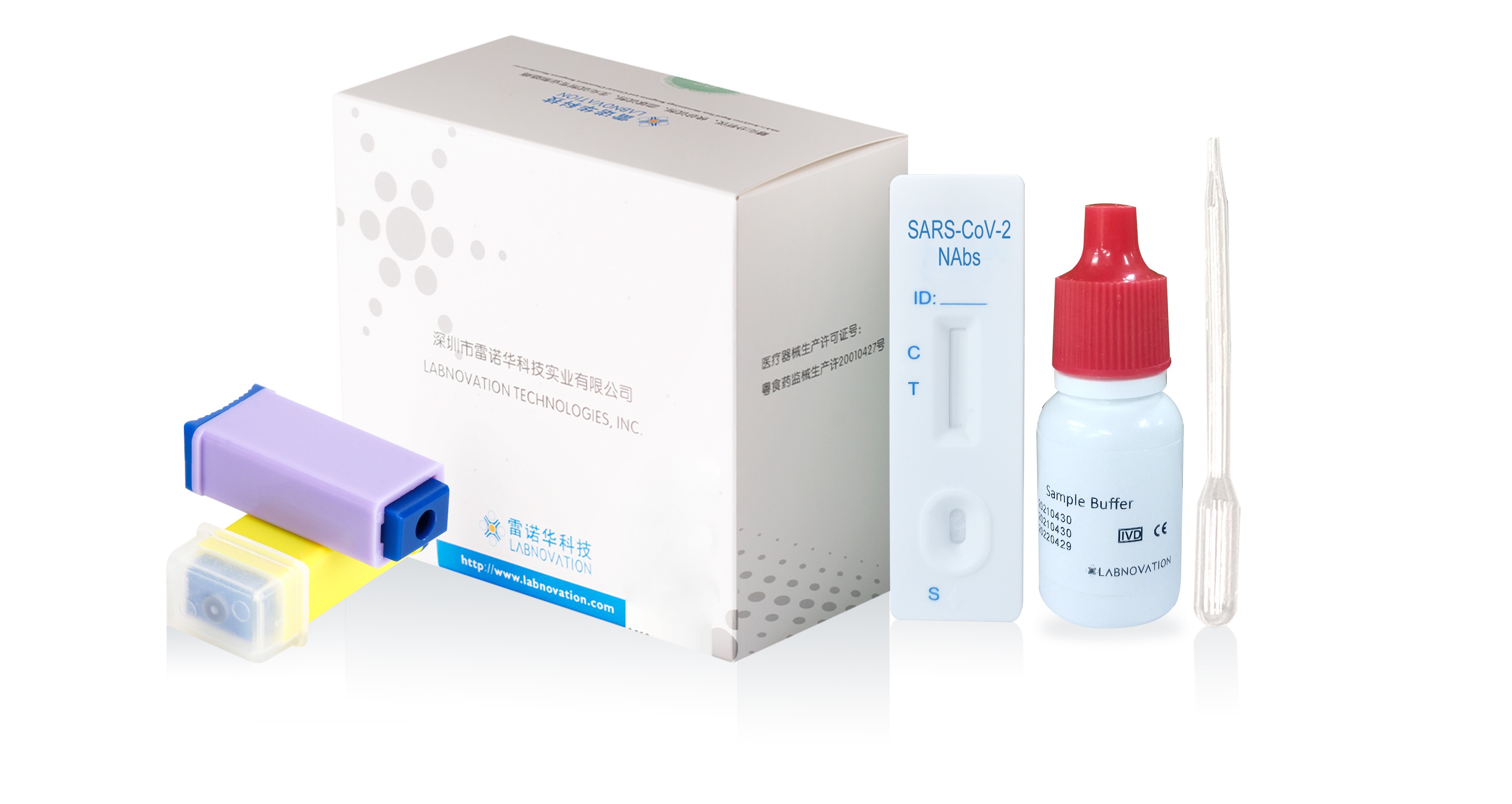
Main Components
- 20 Test cassettes
- 20 Disposable pipettes
- 20 Sterile Safety Lancets
- 1 Sample Buffer
- 1 Colorimetric chart
- 1 Instruction for use
Product Feature
- Fast Test, get antigen test result within 10-15 minutes.
- High sensitivity and specificity
- Easy to operate, no need equipment, convenient and fast
- Require little Specimens

Advantage
- It can not only screen in the window period of infection oneset but
also indicate the previous infection and reduce the rate of missed
diagnosis
- Blood sample detection , simple samplig, simple operation
- Combined with nucleic acid kit to improve the screening rate of
suspected patients
- Rapid screening within 10-15minutes, resukts effective for
15minutes
- Single detection, visual interpretation, no equipment, to adapt to
the community
- Easy operation, compatible with different samples
- Room temperature storage
Notic
- Sample should be human serum, plasma and whole blood only, other
body fluid samples are not tested and may cause incorrect or
inaccurate results.
- All reagents have to be brought to room temperature (18 to 25 °C)
before performing the test.
Use Step
- Place the lanceton the test site.
- Push down the lancet.
- Collect the bloodby transfer pipette.
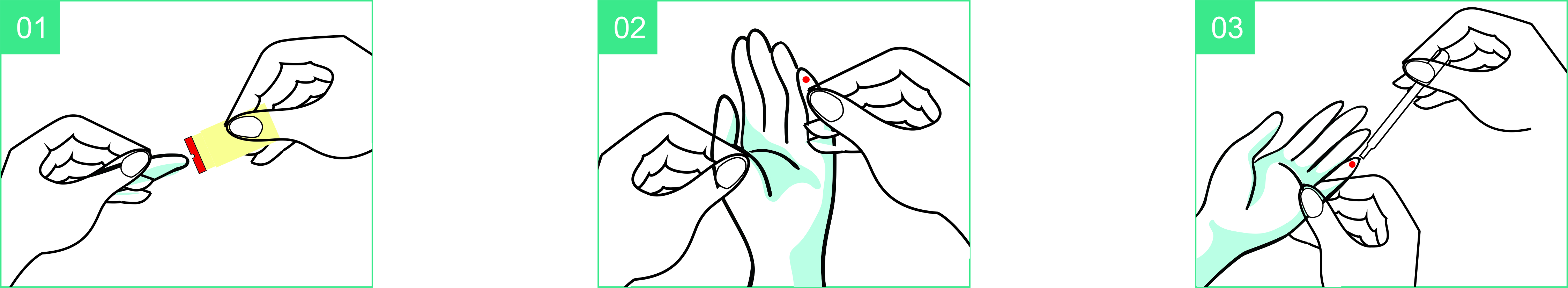
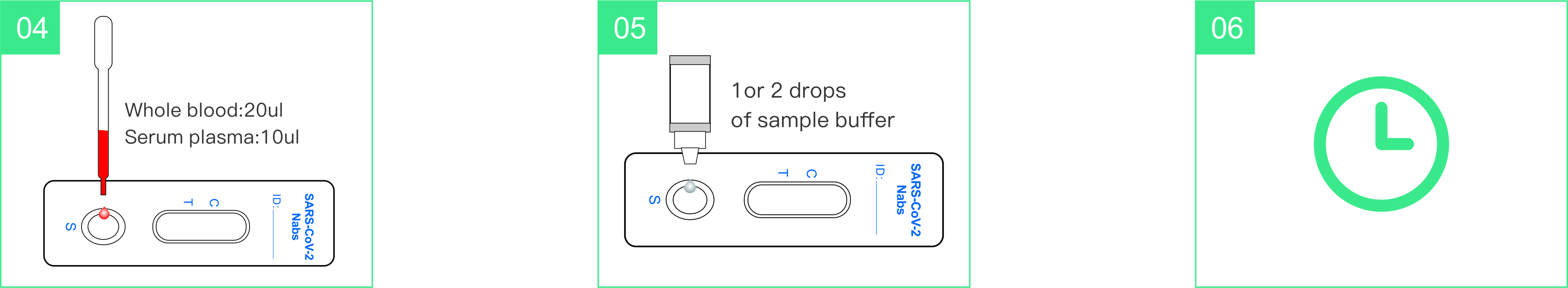
- Add one drop of blood into the sample well.
- Add 1-2 drops (30-60µL) of sample buffer into the sample well
innediately.
- Wait 15 minutes then read the result.
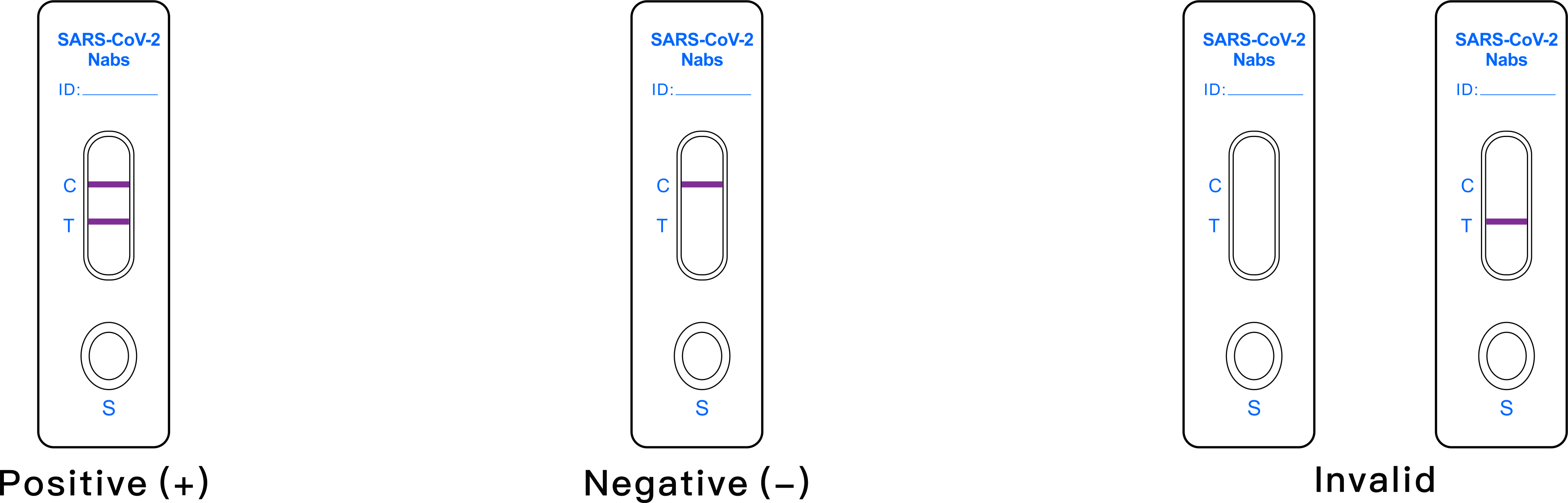
Interpretation of results
- POSITIVE: The T line and C line are both displayed within the
reaction window, indicates the neutralizing antibodies to
SARS-COV-2 are detected. The result is positive.
- NEGATIVE:The C line is displayed within the reaction window only,
indicates absence of neutralizing antibody to SARS-COV-2.The result
is negative..
- INVALID:1. If the control (C) line is not displayed in 15 min, regardless
of whether T line is present, the test result is invalid. It is
recommended that the specimen should be re-tested.
The test result is invalid after 20 min.
LIMITATIONS
- The test is for in vitro diagnostic use only.
- This test is designed for semi-quantitative detection of SARS-COV-2
neutralizing antibodies.
- It is unknown at this time if the presence of antibodies to
SARS-COV-2 confers immunity to re-infection. Consider other
information including clinical history and local disease
prevalence, in assessing the need for a second but different
serology test to confirm an immune response.
- The test results of this kit are for clinical reference only, and
should not be used as the sole basis, and should be combined with
other test methods, as live virus and pseudovirus neutralizing
antibodies assay.
Application
- Hospitals
- Enterprise
- School
- Community
- Family
FAQ
We have the MOQ limit,which is 10000 pieces.
After order confirmed,we will arrange your order immediately,and
offer you an
estimated delivery date.
Business to business account.
We choose Air cargo or Ocean cargo.
- Trade Company or Manufacturer:
Manufacturer




