Labnovation Technologies, Inc. |
|
Antigen Rapid Test Kit SARS-CoV-2 Antigen Rapid Diagnostic Test CE Certificated Best Performance Rapid Test Kit
Intend Use
This kit is an immunochromatography assay which detects SARS-CoV-2 nucleocapsid antigen in the samples with the help of the double antibody sandwich method. These rapid test kit is intended for the qualitative detection of SARS-CoV-2 viral nucleocapsid antigens from human anterior nasal of secretion from individuals suspected of COVID-19.

Product Details
| Item | Value |
| Model Number | LX-401302 |
| Package | 1 Test/Kit |
| Sample volume | 3 Full drops |
| Warranty | 24 Months |
Diagnostic Accuracy
| Name | Specificity | Sensitivity | Total Accuracy |
SARS-CoV-2 Antigen Rapid Test Kit | 100% | 97.45% | 99.17% |
Product Feature

Main Components

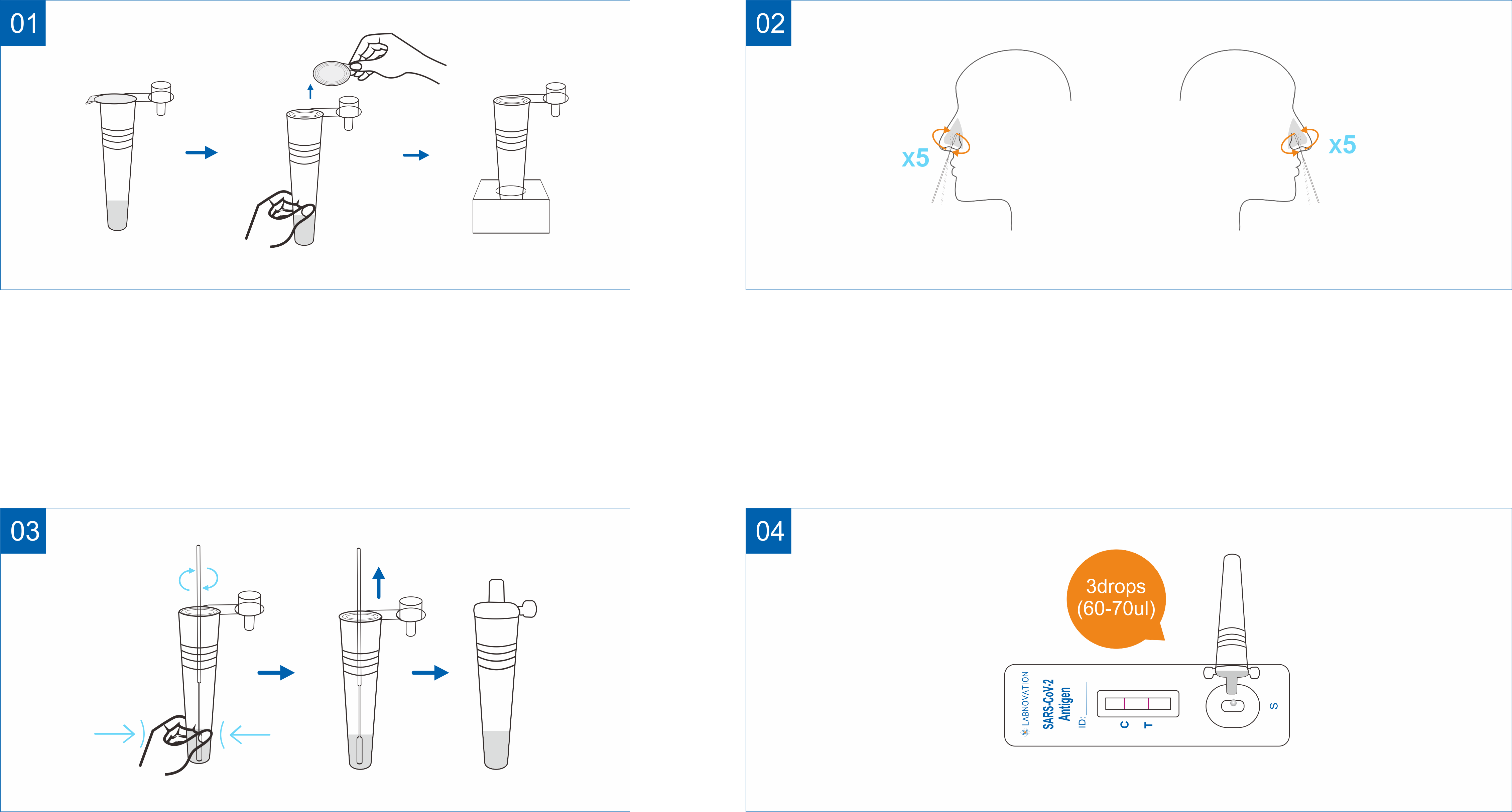
Use Step
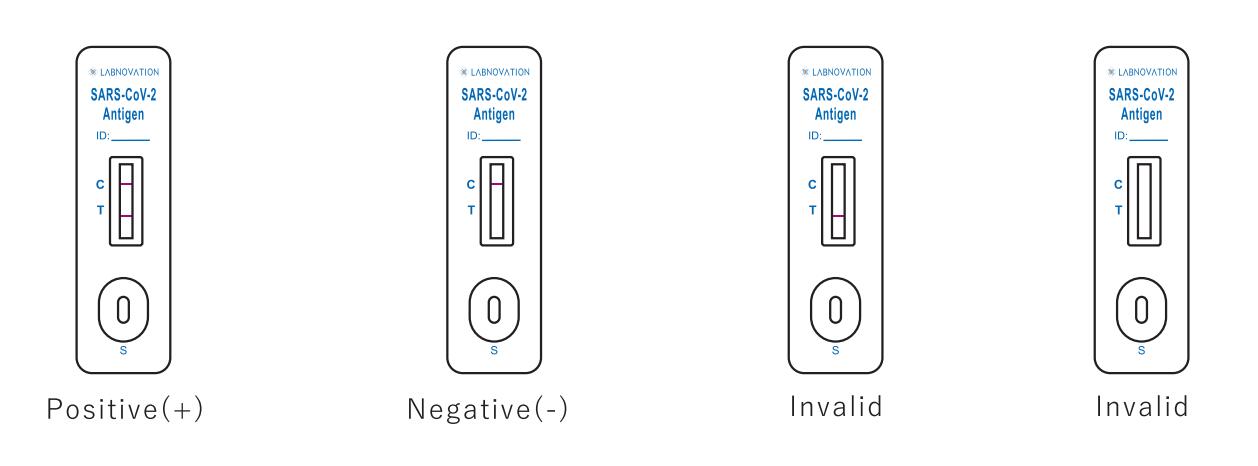
Result Interpretation
POSITIVE: Two colored bands appear on the membrane. One band appears in the control region (C) and another band appears in the test region (T).
NEGATIVE: Only one colored band appears, in the control region (C). No apparent colored band appears in the test region (T).
INVALID: If there is no Control line (C) or only a Test line (T) in the result window, the test did not run correctly and the results are not valid.
Virus Sources
| Global high frequency mutation | Alpha / B.1.1.7(U.K.) | Beta I B.1.351(South Africa) |
| Gemma I P.1(Brazil) | Kappa I B.1.617.1(India) | Delta I B.1.617.2(India) |
| C.37,ect | Alpha I B.1.17(U.K.) | B.1.36.16.etc |
| A.2.5,etc | A.23.1 | Alpha I B.1.17(U.K.) |
| B.1.1.33.etc | C.1.1.etc. | others |
Other Information
• This kit is a qualitative detection, which cannot determine the exact content of antigen.
• The test is intended for use outside the body only.
• Not to be taken internally. Avoid sample buffer contact with skin and eyes.
• Protect from sunlight, do not freeze. Store in a dry place between 2°C and 30°C. Do not use after the expiration date printed on the package.
•Keep out of the reach of children. Any child under age 16 shouldn’t perform the test without parental guidance, or professional aid.
• Not following the exact instructions can affect the outcome of the test. The final diagnosis must be confirmed by a physician.
• Do not use the test if the packaging is damaged. Do not use broken test components.
• All test components are only intended to be used for this test. Do not reuse the test or test components.
• The test should be carried out immediately or within one hour after opening the foil pouch (10°C -30°C, humidity <60%).
• Samples be processed as soon as possible after sample collection. If the test cannot be performed immediately, the sample should be stored in a sealed state, stored at 2~8°C for 8 hours, and stored below -20°C for 1 month. Long-term storage is not recommended.
• Poor vision, color blindness or poor lighting may affect your ability to interpret the test correctly.
• DISPOSAL The test kit can be disposed of with normal household waste in accordance with applicable local regulations.
• A negative result does not rule out the infection of a SARS-CoV-2 infection. Therefore, the test should not be used as the only reference for the clinical diagnosis. The result must be confirmed by the PCR.
• After use, rinse hands or, in case of contact with the buffer solution, the affected body parts thoroughly with water.
• If symptoms persist: Seek medical advice.
Certificate
