DE Medical Technology Jiangsu Co.,Ltd |
|
IVD Infections disease diagnostic Hepatitis B Virus combo test kit
HBsAg ,HBsAb,HBeAg ,HBeAb, HBcAb 5 in 1 test panel HBV combo test
card
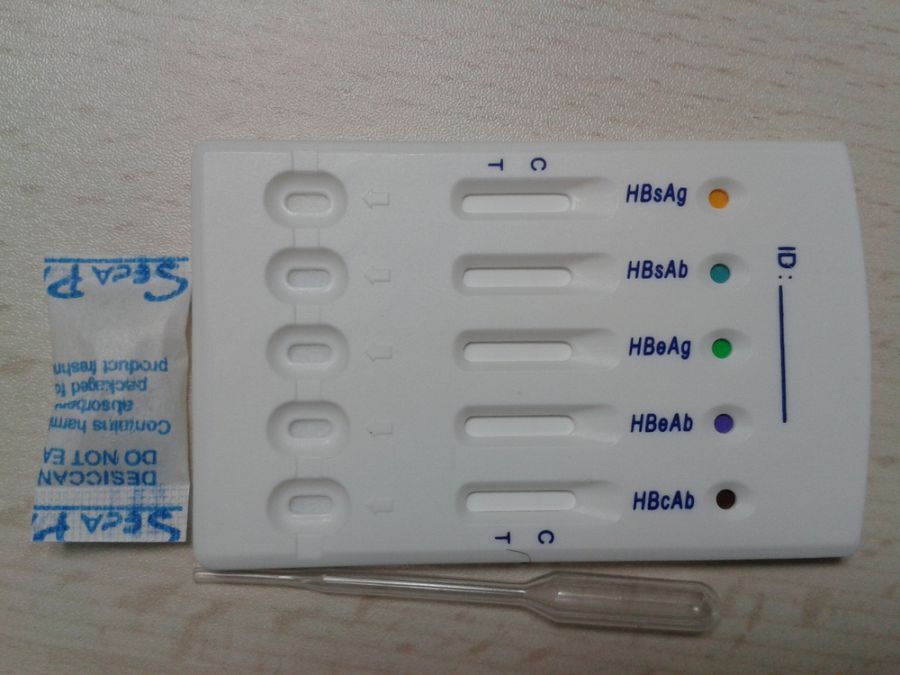

Introduction
HBV 5in1 combo Rapid Test Kit for whole blood/serum/plasma is
tended for the screening of blood ,safe, rapid, accurate, easy to
use, easy to read.
The HBV 5in1 Rapid Test is a chromatographic immunoassay (CIA) for
the rapid qualitative determination of human hepatitis B virus(HBV)
in whole blood.This test is intended for the screening of blood and blood products
to be used for transfusion and an aid for the diagnosis of existing
or previous hepatitis B in one panel.
Compositions
HBsAg test strip: anti-HBsAg monoclonal antibody A (solid gold
standard material), anti HBsAg monoclonal antibody B (test line),
goat anti-mouse IgG (control line).
HBsAb test strip: Purified HBsAg (solid gold standard material),
purified HBsAg (test line), HBsAg monoclonal antibody (control
line).
HBeAg test strip: anti-HBeAg monoclonal antibody A (solid gold
standard material), anti-HBeAg monoclonal antibody B (test line),
goat anti-mouse IgG (control line).
HBeAb test strip: anti-HBeAg monoclonal antibody A (solid gold
standard material), anti-HBeAg monoclonal antibody B (test line),
goat anti-mouse IgG (control line).
HBcAb test strips: Recombinant HBcAg (solid gold standard
material), anti-HBcAg monoclonal antibody A (test line),
recombinant HBcAg monoclonal antibody (control line).
Requirements on Specimen
1. Serum, plasma, whole blood samples collected by venous
conventional methods, test samples must be collected with a clean
container, can be used anticoagulant heparin, EDTA, sodium
citrate,Sodium oxalate
2. After collection separate the serum and plasma as soon as
possible, in order to avoid hemolysis. Use fresh specimens.
3. Serum, plasma, whole blood samples stored at 2-8 °C in
refrigerator preferably not
more than 3 days, if not tested immediately should be stored at -20
°C.
Test Procedure
1. Remove the bag from the original packaging (Note: Open the bags
should be preceded by the reagents and samples to room temperature.
use it in 1 hour (20% -90% humidity, temperature:10 °C -50 °C)
2. Open the sealed foil pouch; remove the test panels, flat on the
horizontal table number (corresponding with the specimen)
3. Drop (about 50ul) sample in the detection of sample holes on the
card, and then drop two drops specimen buffer to each well.
4. 15-20 minutes of observation, the results no clinical
significance after 20 minutes.
Result Judgment
HBsAg HBsAb HBeAg:
Positive: Two red band appears in the test area (T), another in the
control area(C). The positive results show that: samples containing
analysts.
Negative: Only control line (C) a red band appears in the detection
zone (T) without the red band appears. The negative results show
that: samples don’t containing analysts.
Invalid: Quality control area (C) does not appear red band,
indicating that the operator error or reagent failure.
HBeAb HBcAb:
Positive: Only control line (C) a red band appears in the detection
zone (T) without the red band appears. The positive results show
that: samples containing analysts. If the quality control area C
has a red band, the test area T has a very weak red band, which is
called weakly positive, indicating that the samples contain a small
amount of antibodies.
Negative: Two red band appears in the test area (T), another in the
control area(C). The negative results show that: samples don’t
containing analysts.
Invalid: Quality control area (C) does not appear red band,
indicating that the operator error or reagent failure.
Performance indicators
The detection limit of three subtypes of HBsAg (ADR, ADW, AY) is
not more than 2.5NG/ML The detection limit of HBsAg not higher than
30.0mlu/ml.
| positive coincidence rate | negative coincidence rate | |
| HBeAg | 98.71% | 100% |
| HBeAb | 99.35% | 99.86% |
| HBcAb | 99.83% | 99.50% |
Attentions
1. Do not uses test kit beyond expiry date. Please check the
package and the contents are complete before use, if damaged,
cannot be used.
2. Serum, plasma, whole blood specimens were stored with the
refrigerator 2-8 °C best not more than 3 days, if not timely
detection then -20 °C frozen and the specimen is mixed melting and
to avoid repeated freezing and thawing.
3. Test line color intensity level of the sample are not
necessarily linked in the antibody titer, Positive results cannot
be confirmed as a basis before further confirmatory testing done.
4. The strength of control line does not represent the quality of
reagents, the reagent effective as long as its color clearly
visible.
5. If the filtration speed is very slow or evens no filtration,
please retreat the specimen and test again.
Storage and Expiry
Room temperature4-30 °C, avoid hot and sunshine, dry place, not
frozen, valid for 24 months.