1. This package insert must be read completely before performing
the test. Failure to follow directions in package insert may yield
inaccurate test results.
2. For professional in vitro diagnostic use only. Do not use after
expiration date.
3. Do not eat, drink or smoke in the area where the specimens or
kits are handled.
4. Do not use test if pouch is damaged.
5. Handle all specimens as if they contain infectious agents.
Observe established precautions against microbiological hazards
throughout in the collection, handling, storage, and disposal of
patient samples and used kit contents.
6. Wear protective clothing such as laboratory coats, disposable
gloves and eye protection when specimens are assayed.
7. Viral Transport Media (VTM) may affect the test result, do not
store specimens in viral transport media; extracted specimens for
PCR tests cannot be used for the test.
8. Wash hands thoroughly after handling.
9. Please ensure that an appropriate amount of samples are used for
testing. Too much or too little sample size may lead to deviation
of results.
10.The used test should be discarded according to local
regulations.
11.Humidity and temperature can adversely affect results.
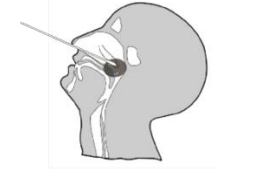
Specimen Collection
1. Insert a sterile swab into the nostril of the patient, reaching
the surface of the posterior nasopharynx.
2. Swab over the surface of the posterior nasopharynx.
3. Withdraw the sterile swab from the nasal cavity
DIRECTIONS FOR USE
Allow the test, extracted specimen and/or controls to equilibrate
to room temperature (15-30°C) prior to testing.
1. Remove the test from the sealed foil pouch and use it within one
hour. Best results will be obtained if the test is performed
immediately after opening the foil pouch.
2. Invert the specimen collection tube and add 3 drops of extracted
specimen to each of the specimen well(S) respectively and then
start the timer.
3. Wait for the colored line(s) to appear. Read the result at 15
minutes. Do not interpret the result after 20 minutes.

SUMMARY
The novel coronaviruses belong to the β genus. COVID-19 is an acute
respiratory infectious disease. People are generally susceptible.
Currently, the patients infected by the novel coronavirus are the
main source of infection; asymptomatic infected people can also be
an infectious source. Based on the current epidemiological
investigation, the incubation period is 1 to 14 days, mostly 3 to 7
days. The main manifestations include fever, fatigue and dry cough.
Nasal congestion, runny nose, sore throat, myalgia and diarrhea are
found in a few cases. Influenza (commonly known as ‘flu’) is a
highly contagious, acute viral infection of the respiratory tract.
It is a communicable disease easily transmitted through the
coughing and sneezing of aerosolized droplets containing live
virus. Laboratory identification of human influenza virus
infections is commonly performed using direct antigen detection,
virus isolation in cell culture, or detection of influenza-specific
RNA by reverse transcriptase-polymerase chain reaction (RT-PCR).
Rapid tests for influenza A and B virus infections, which can
provide results within 30 minutes.
Respiratory Syncytial Virus (RSV), which causes infection of the
lungs and breathing passages, is a major cause of respiratory
illness in young children. In adults, it may only produce symptoms
of a common cold, such as a stuffy or runny nose, sore throat, mild
headache, cough, fever, and a general feeling of being ill.Most
children with RSV infection, both those who were hospitalized and
those who were treated as outpatients, had no coexisting medical
conditions or characteristics that significantly identified them as
being at greater risk for severe RSV disease, except for being
under 2 years of age.

