Henan Lantian Medical Supplies Co.,Ltd. |
|
Cardiac troponin I/myoglobin/creatine kinase isoenzyme (cTnI/myo/CK-MB) includes the detection of the following three indicators: cardiac troponin I (cTnI), myoglobin (myo), creatine kinase isoenzyme (CK-MB). The combined detection of these three indicators is of great significance in the early diagnosis of acute myocardial diseases such as acute myocardial infarction (AMI).
American Academy of Clinical Biochemical Sciences (Nacb) practice guide
Myocardial necrosis markers should be detected in all suspected ACS
patients
Troponin is the first choice for the diagnosis of myocardial
infarction
Myoglobin combined with specific markers of myocardial necrosis
(such as cTnI/CK-MB) is helpful to more quickly exclude or diagnose
myocardial infarction
| MYO | CK-MB | cTnI | |
| molecular weight | 17.8KD | 86KD | 22.5KD |
| Rise time | 1-2h | 4-6h | 3-6h |
| Peak time | 6-9h | 24h | 12-16h |
| recovery time | 18-30h | 2-3d | 4-10d |
| Clinical value | 1. The most important index of AMI early exclusion 2. The best index of AMI recurrence diagnosis and reperfusion treatment effect evaluation | 1. Estimate infarct size or reinfarction 2. The most valuable marker of non ST segment elevation MI | 1. The first choice marker for AMI diagnosis 2. Determine the infarct size and make risk stratification |
| Possibility of outcome | |||
| cTnI | CK-MB | MYO | Clinical possibility |
+ | + | + | Myocardial infarction / myocardial injury can be diagnosed basically, and the occurrence time is within 12-24 hours; |
| + | + | - | Basically, it can be diagnosed as myocardial infarction / myocardial injury, and the detection time is more than 1 day, which has passed the detection window period of myo; |
| + | - | - | Myocardial infarction / myocardial injury has occurred for 2-3 days, it is recommended to reexamine after a few hours; |
| + | - | + | In patients with myocardial infarction / myocardial injury, the detection time may be more than 3 days, and the patients may have re injury or enlarged infarct size; |
| - | + | + | For early myocardial infarction / myocardial injury / muscle injury, cTnI should be reexamined within 4-8 hours; |
- | - | + | 1. Early myocardial infarction / myocardial injury, cTnI should be reexamined within 4-8 hours; |
| 2. It may be caused by other injury diseases, such as creation, skeletal muscle injury, liver and kidney disease; | |||
| - | + | - | 1. Minor myocardial injury, cTnI should be reexamined within 4-8 hours; |
| 2. It may be a non myocardial injury disease, and the detection time has passed the myo window; | |||
- | - | - | Myocardial infarction / myocardial injury is basically ruled out. If it is highly suspected, reexamination is recommended every 2-4 hours. |
| Detection range | sample size | Sample type | Test time | ||
| cTnI | MYO | CK-MB | 60μl | Whole blood / serum / plasma | 15min |
| 0.1~50ng/mL | 10~200ng/mL | 0.8~100ng/mL | |||
Outpatient, emergency, physical examination center, cardiovascular, ICU, chest pain center and other departments
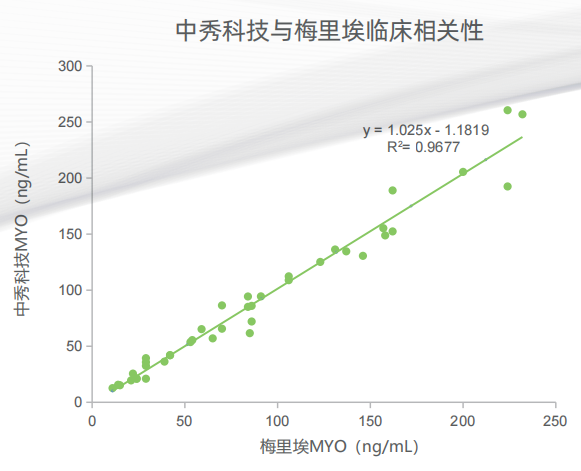
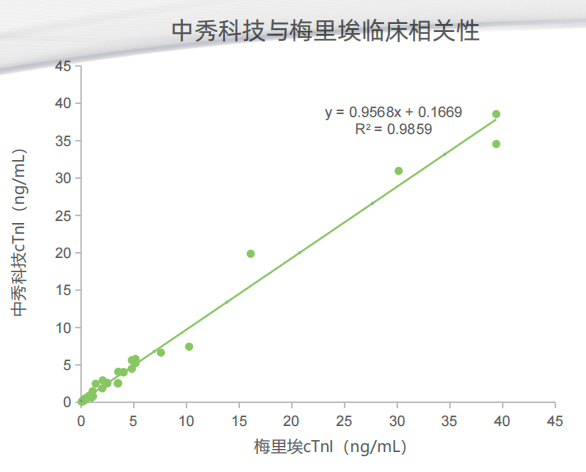
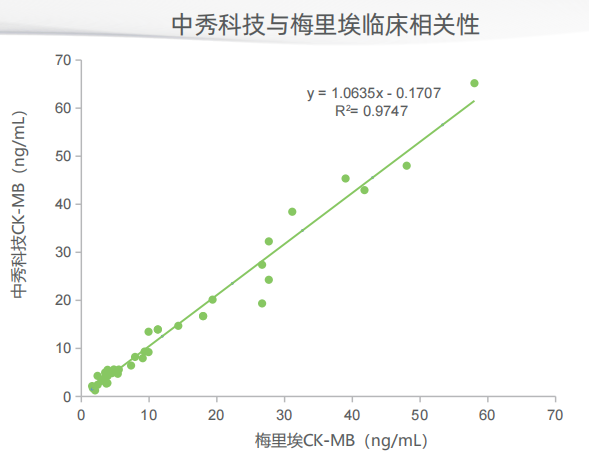




Zhongxiu Science And Technology Co.,Ltd., is a high-tech enterprise
engaged in the research and development, production and operation
of in vitro diagnostic products. The in vitro diagnostic products
developed by the company cover POCT series, microbial series,
biochemical series and immune series reagents and supporting
instruments.
The company has always adhered to the core concept of "fast and
accurate, living up to life", committed to providing society with
excellent products and services, and contributing to the cause of
human health.