*Rapid Test Kit Blood Test checks if you have had COVID-19 and if
you have developed antibodies to fight the virus
*Immediate results within 10-15 minutes - significant time and cost
savings over lab methods
*Easy to use and read - no specialist equipment needed
*Cassette test using a blood, serum, or plasma sample
*CE Certified
DIRECTIONS FOR USE
Allow the test device,specimen,buffer,and/or controls to reach
room temperature (15-30°C) prior to testing.
1. Bring the pouch to room temperature before opening.Remove the test device from the sealed pouch and use it as soon as possible.
2. Place the test device on a clean and level surface.
3. Add 20μl whole blood or 10μl serum and plasma into the sample well of the test device, then add 80μl buffer into the
sample well.
4. Read the result at 15~20 minutes. The result is invalid after 20 minutes.
Note: 1 drop volume of the disposable plastic pipette provided is about 10μl.
INTERPRETATION OF RESULTS
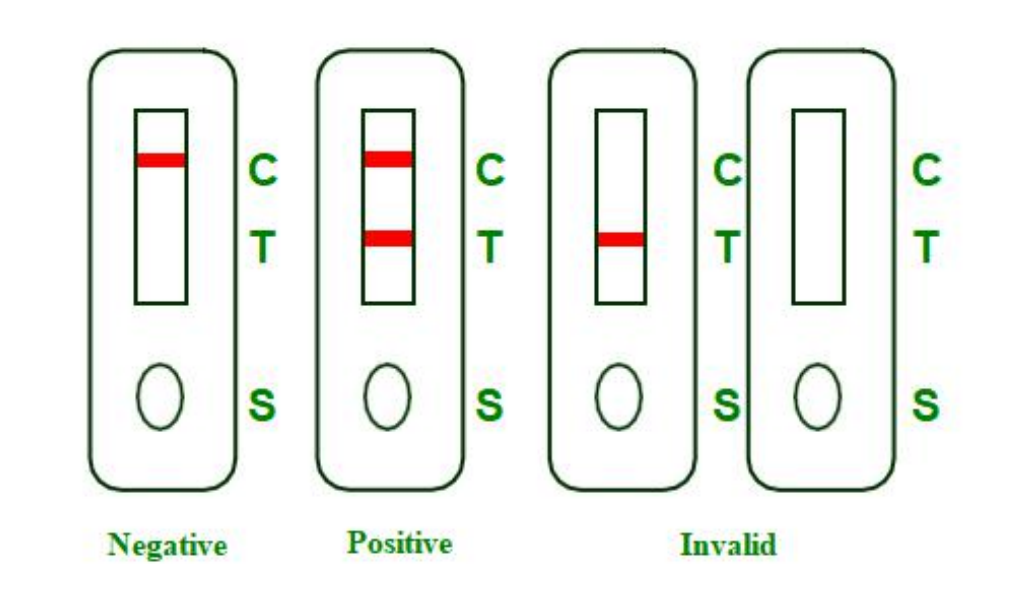
NEGATIVE:Presence of a single colored band in the control region (C) indicates the absence of COVID-19 neutralizing antibodies or that the concentration of antibodies in sample is below the detection cut-off level.
POSITIVE: Presence of two visible, pink-colored bands, one in the control region (C) and one in test region (T), indicates presence of COVID-19neutralizingantibodies in sample.
INVALID: There is no line appeared in the C region. Insufficient buffer volume or incorrect procedural techniques are the most likely reasons for control line failure.Review and repeat the procedure with a new test device. If the problem persists,discontinue using the test kit immediately and contact your local distributor.