Labnovation Technologies, Inc. |
|
SARS-CoV-2 Antigen Rapid Test Kit (Immunochromatography)
Intend Use
Product Details
| Item | Value |
| Brand | Labnovation |
| Model Number | LX-401301 |
| Packaging | 20 Tests |
| Warranty | 24 Months |
| Power Source | Package Insert |
| Quality Certification | CE, MSDS |
| Safty Standard | ISO13485 |
| After-sale Service | Online technical support |
| Specimen | nasopharyngeal swabs and oropharyngeal swabs |
| Sample volume | 3 Full drops |
| Test Speed | Within 15 minutes |
Product Feature

PRINCIPLE

Main Components
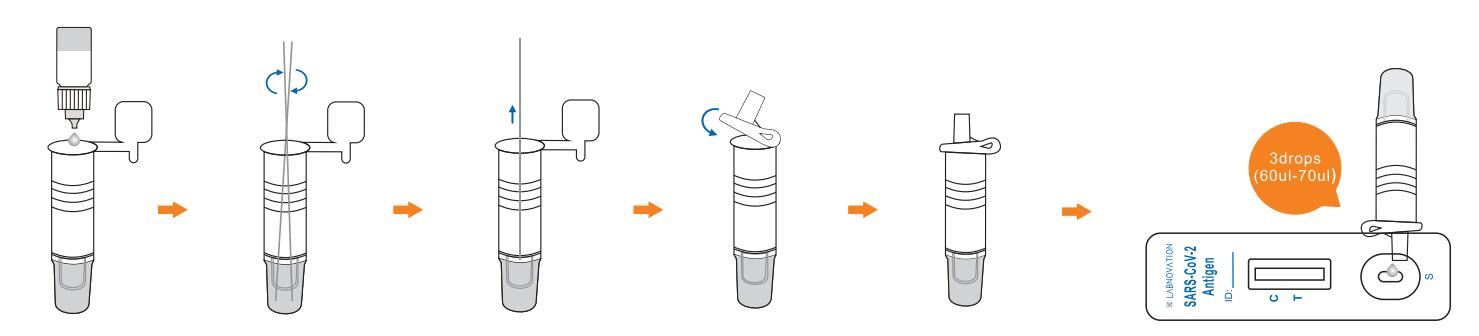
Use Step
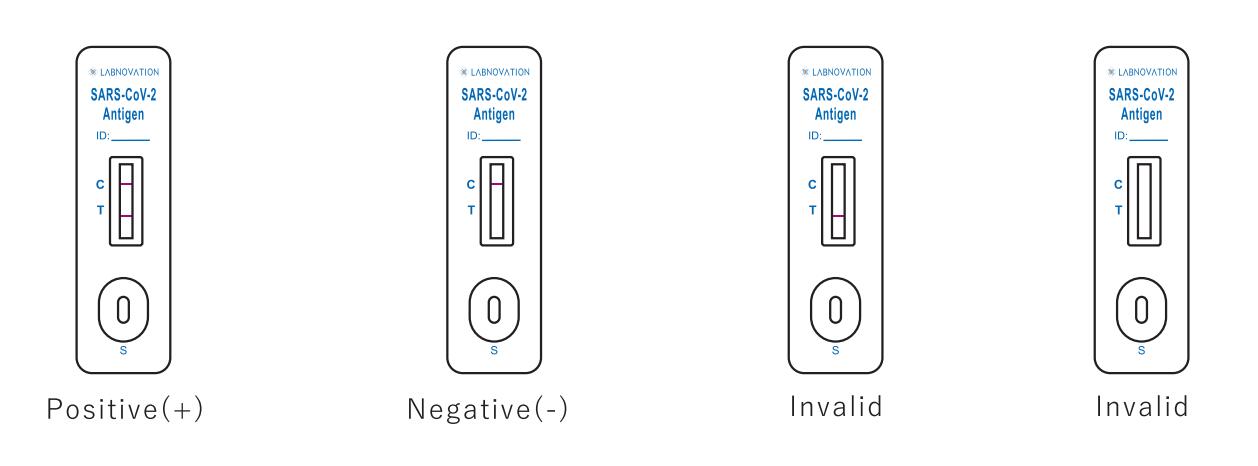
Result Interpretation
POSITIVE: Two (2) distinct colored lines appear. One line should be in the control region (C) and the other line should be in the test region (T).
NEGATIVE: One (1) colored line appears in the control region(C). No apparent colored line appears in the test region (T).
INVALID: No colored lines appear, or control line fails to appear, indicating that the operator error or reagent failure.
Virus Sources
| Global high frequency mutation | Alpha / B.1.1.7(U.K.) | Beta I B.1.351(South Africa) |
| Gemma I P.1(Brazil) | Kappa I B.1.617.1(India) | Delta I B.1.617.2(India) |
| C.37,ect | Alpha I B.1.17(U.K.) | B.1.36.16.etc |
| A.2.5,etc | A.23.1 | Alpha I B.1.17(U.K.) |
| B.1.1.33.etc | C.1.1.etc. |
Certificate