Rapid Antigen Test Kit SARS-CoV-2 Antigen Rapid Test Kit Individual
Self Testing Rapid Test Kit
Intend Use
- The SARS-CoV-2 Anigen Rapid Test kit is for in vitro diagnostic use
only
- The rapid test kit is for individual self testing use
- Immunochromatography assay
- This rapid test kit is intended for the qualitative detection of
SARS-CoV-2 viral nucleocapsid antigens from human anterior nasal of
secretion from individuals suspected of COVID-19.
- Test result should not be used as the sole basis for
treatment.
- Further nucleic acid detection should be carried out for suspected
population whose antigen test result is positive or negative.
Product Details
| Item | Value |
| Model Number | LX-401302 |
| Package | 1 Test/Kit |
| Specificity | 100.00% |
| Sensitivity | 97.45% |
| Total Accuracy | 99.17% |
| Sample Type | Nasopharyngeal Sample |
| Sample volume | 3 Full drops |
| Test Time | < 15 minutes |
| Warranty | 24 Months |
| Quality Certification | CE, MSDS |
| Safty Standard | ISO13485, ISO9001 |

Product Feature
- Results ready in 15minutes
- Accurate diagnostic tool
- Easy to administer and read results
- Affordable, no need for instrument, highly portable
- Single Pack more protable and convenient
- Individual Self Testing At Home

Main Components
- Test Cassette
- Sample Tube
- Sample Extraction Buffer
- Swab
- Instruction for use

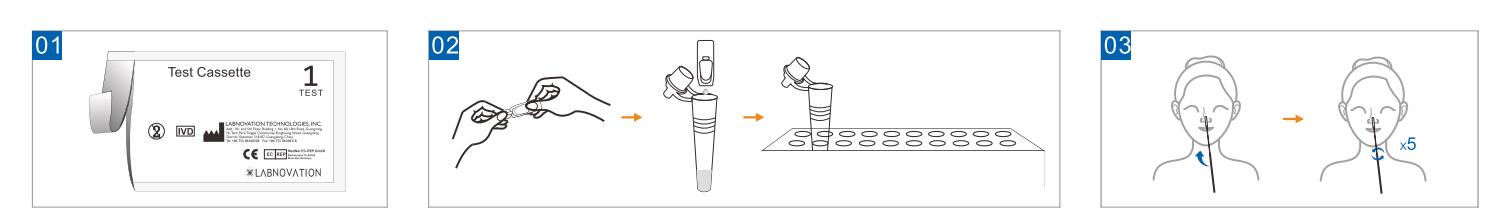
Use Step
- Open the sealed pouch and remove the test casstette. Lay it face up
on a clean, dry and flat surface.
- Unpack the sample extraction, add all of the sample extraction into
the sample tube and then put the tube into tube stand.
- Gently, insert the entire absorbent tip of the swab (around 1.5 cm)
into your nostril. Rotate walls of your nostril 5 times or more.
Use the same swab to repeat steps in the other nostril.
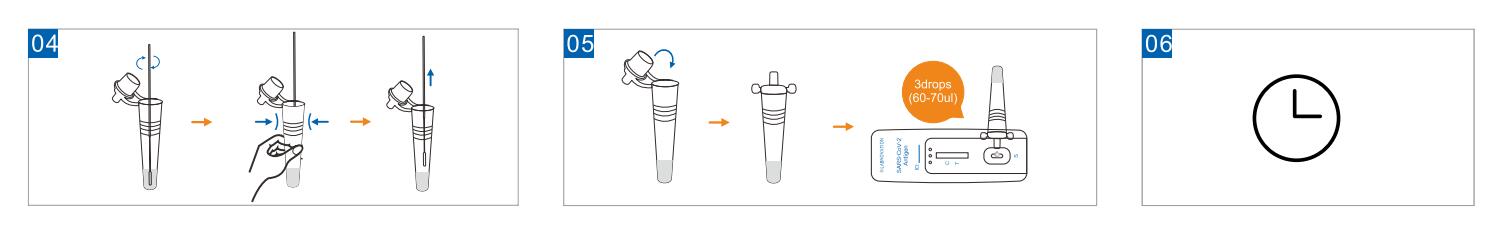
- Insert the swab into the ssample tube with extraction buffer. Mix
well. Mix well and squeeze the swab 10-15 times by compressing the
walls of the tube against the swab. Roll the swab head against the
inner wall of the tubes as you remove it.
- Close the cape of the sample tube. Add 3 full drops of the mixed
solution vertically into the sample well(S) of the test cassette.
- Read the result 15-20minutes after adding the sample. Result got
after 20 minutes is invalid.
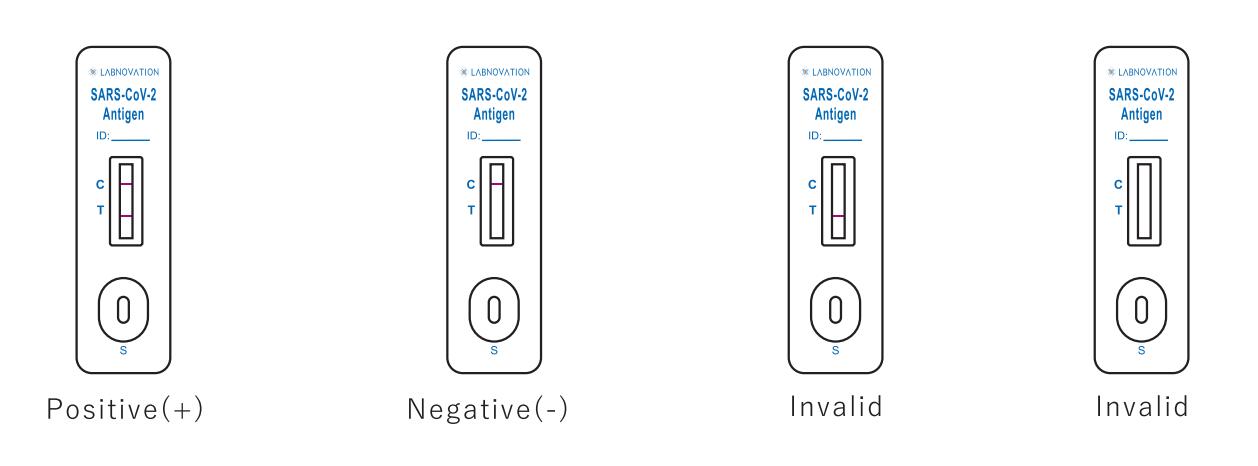
Result Interpretation
POSITIVE: Two colored bands appear on the membrane. One band appears in the
control region (C) and another band appears in the test region (T).
NEGATIVE: Only one colored band appears, in the control region (C). No apparent colored band appears in the test region (T).
INVALID: If there is no Control line (C) or only a Test line (T) in the result window, the test did not run correctly and the
results are not valid.
Virus Sources
| Global high frequency mutation | Alpha / B.1.1.7(U.K.) | Beta I B.1.351(South Africa) |
| Gemma I P.1(Brazil) | Kappa I B.1.617.1(India) | Delta I B.1.617.2(India) |
| C.37,ect | Alpha I B.1.17(U.K.) | B.1.36.16.etc |
| A.2.5,etc | A.23.1 | Alpha I B.1.17(U.K.) |
| B.1.1.33.etc | C.1.1.etc. | |
Other Information
- This kit is a qualitative detection, which cannot determine the
exact content of antigen.
- The test is intended for use outside the body only.
- Not to be taken internally. Avoid sample buffer contact with skin
and eyes.
- Protect from sunlight, do not freeze. Store in a dry place between
2°C and 30°C. Do not use after the expiration date printed on the
package.
- Keep out of the reach of children. Any child under age 18 shouldn’t
perform the test without parental guidance, or professional aid.
- Not following the exact instructions can affect the outcome of the
test. The final diagnosis must be confirmed by a physician.
- Do not use the test if the packaging is damaged. Do not use broken
test components.
- All test components are only intended to be used for this test. Do
not reuse the test or test components.
- The test should be carried out immediately or within one hour after
opening the foil pouch (15-30°C, humidity <60%).
- Samples be processed as soon as possible after sample collection.
If the test cannot be performed immediately, the sample should be
stored in a sealed state, stored at 2~8°C for 8 hours, and stored
below -20°C for 1 month.
- Long-term storage is not recommended.
Certificate







