Huachenyang (Shenzhen) Technology Co., Ltd |
|
Verified Suppliers
|
|
COVID-19 IgG IgM Antigen Rapid Test Device With NMPA Certificate
COVID-19 IgG/IgM Rapid Testing Kit SARS-CoV-2 Rapid Test IgG/IgM Rapid Test Cassette
product description:
COVID-19 Antigen Test Kit (Lateral Chromatography) is an immunochromatographic assay for the rapid, qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 in nasopharyngeal(NP) and nasal(NS) swab specimens directly or after the swabs have been added to viral transport media from individuals who are suspected of COVID-19 by their healthcare provider, Testing is limited to certified laboratories that meet the requirements to perform moderate, high or waived complexity tests. This test is authorized for use at the Point of Care (POC), i.e., inpatient care settings operating underlie Certificate of waiver,Certificate of Compliance,or Certificate of Accreditation. The test is for the identifica- tion ofSARS-CoV-2 nucleocapsid antigen that is generally detectable in upper respiratory samples during the acute phase of infection. Positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status, Positive results do not rule out bacterial infection or co-infection with other viruses.
Negative results should be treated as presumptive, do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. Negative results should be considered in the context of a patients recent exposures, history and the presence of clinical signs and symptoms consistent with COVID-19,and confirmed with a molecular assay, if necessary, for patient management.
COVID-19 Antigen Test Kit(Lateral Chromatography) is intended for use by trained clinical laboratory personnel specifically instructed and trained in the techniques of in vitro diagnostic procedures, and proper infection control procedures and individuals similarly trained in point ofcare settings.
Technical features:
This reagent uses double antibody sandwich method to legally detect the novel coronavirus antigen in nasopharyngeal and oropharyngeal swabs. During the detection process, the gold-labeled anti-coronavirus monoclonal antibody on the labeling plate binds to the neocoronavirus antigen in the sample to form a complex, and the reaction complex moves forward along the nitrocellulose membrane under the action of chromatography, passing through the nitrocellulose The detection area (T) on the plain film is pre-coated with anti-2019-nCoV monoclonal antibody, and finally a red reaction line is formed in the T area. If the sample does not contain 2019-nCoV antigen, a red reaction line cannot be formed in the T zone. Regardless of whether the test sample contains 2019-nCoV antigen, a red reaction line will always be formed in the quality control area (C).
Ordering Information:
CY-F006-AG25 (25 servings/box)
Product use:
Application: For suspicious patients with symptoms, mild symptoms, or even without symptoms, also for testing people with close contact with infected patients and people under quarantine control.
* Utilizes human whole blood, serum, or plasma
* Used in rapid, qualitative, and differential detection of IgG and IgM antibodies
* Delivers clinical results between 2 and 10 minutes
* Visual interpretation of results
* No special equipment needed
product instructions:
SAMPLE COLLECTION AND PREPARATION Set up for test
Remove one extraction tube and one COVID-19 Antigen Test Kit(Lateral Chromatography) cassette from its foil pouch immediately before testing
Label the test cassette and the extraction tube for each specimen to be tested. Place the labeled extraction tube(s) in a rack in the designated area of the workspace. See illustration 1.
Nasal Swab Specimen Collection:
To collect a nasal swab sample, carefully insert the swab(supplied in the kit) into the nostril that presents the most secretion under visual inspection.Using gentle rotation, push the swab until resistance is met at the level of the turbinates(about 2.0-2.5cm or near one inch into the nostril). Roll the swab about 5 times against the nasal wall then remove it from the nostril.
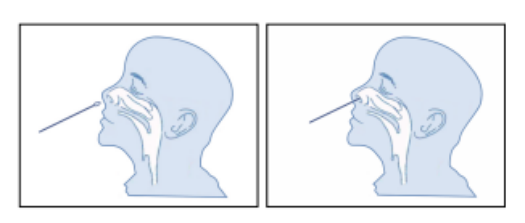
Sample Preparation:
Unscrew the cap of the sample extraction buffer bottle, squeeze the sample extraction bufter bottle and add the sample extraction buffer into the extraction tube.(See illustration 2) After the specimen collection is done, immediately immerse the swab into the sample extraction bufter.(See illustration 3) In order to make the sample extracting completely permeated intc the buffer from swab,rotate the swab against the tube wall repeatedly for 10 seconds and then use fingers to hold the tube (the tube wall is semi-solid) to squeeze the swab for several times while slowly remove the swab out of the tube.(See illustratior4)The purpose of swab squeezing against the tube wall is important, because it can keep the specimen containing liquid to remain in the tube as much as possible. After taking out the swab and discarding it into a biohazard waste container, put on a nozzle (supplied in the kit) to the top of the extraction tube tiehtly and gently shake the tube to mix the inside liquid well See illustration 5.

RESULTINTERPRETATION:
Positive Result:Both the Test"T" position and Control "C" position appear visible color lines, If the test "T" position shows a faint color, the result is also positive.
Negative Result:Only the Control "C" position appears color, while the test "T" position is blank.
Invalid Result:If the control "C" remains blank without color appearing, the test result is invalid because the detecting function is failed to work. Thus, retest the specimen is required.
PERFORMANCE CHARACTERISTICS:
Sensitivity:82.14%(23/28), in comparison with nucleic acid amplification test, confirmed positive cases. Specificity: 99.45%(180/181), the negative cases were also confirmed by nucleic acid amplification test.
Cross-reactivity: There is no cross-reactivity with influenza A virus, influenza B virus, adenovirus, Coxsackie virus, ECHO virus, and enterovirus; no cross-reactivity with Chlamydia pneumoniae, Mycoplasma pneumoniae, Chlamydia psittaci, and Chlamydia trachomatis; no cross-reactivity with Acinetobacter baumannii, Bordetella pertussis, Candida albicans, Escherichia coli, Haemophilus influenzae and Neisseria gonorrhea.
| model | CY-F006-AG25 |
| store | Inside the sealed bag |
| Validity period | 24 months |
| Accessory 1 | detection box |
| Accessory 2 | Sampling swab |
| Accessory 3 | sample buffer |
| Place of production | China |
| brand | HUACHENYANG |
PRECAUTIONS:
1. This kit is only used for in vitro auxiliary diagnosis and should be used strictly in accordance with the Instructions For Use.
2. Please check the effective date and package integrity of the kit before use. If the package of the test device is damaged anc expires, it cannot be used.
3. The time for testing and results reading must be followed.
4. After the pouch of the test cassette is opened, the test should be performed within 60 min. The test cassette can only be used once.
5. The kit is stored at 2 C~30C. Keep away from moist, sunlight, heat, or freezing conditions.
6. The test results of this kit are only for clinical reference, and the clinical diagnosis of the disease should be considered in combination with the patient's symptoms, signs, medical history, other laboratory tests, and treatment response.