WWHS Biotech.Inc(exclusive marketed by Dawin) |
|
Verified Suppliers
|
|
【Product name】
D-Dimer Rapid Quantitative Test(Fluorescence immunoassay)
【Package specification】
20 Tests/box
【Intended use】
This kit is used for quantitative determination of D-dimer in human whole blood and plasma.
【Inspection principle】
The principle of immunofluorescence chromatography was applied to the kit. The D-dimer antigen in the sample was first bound with the conjugated compound of fluorescent labeled D-dimer monoclonal antibody, then moved and combined with another D-dimer monoclonal antibody fixed on the nitrocellulose membrane, and the double antibody sandwich complex was formed at the detection line of the cellulose nitrate membrane. The quantitative detection results were obtained by NIR-1000 dry fluoroimmunoassay analyser.
【Main components】
| Name | Quantity | Component |
| Test card | 25 | It is composed of fluorescent pad (coated with fluorescent labeled D-dimer monoclonal mouse antibody), nitrocellulose membrane (coated with D-dimer monoclonal mouse antibody and Goat anti mouse IgG antibody), absorbent paper and backing |
| Sample buffer | 25(300μL/tube) | Phosphate buffer |
| ID card | 1 | Record the standard curve information of this kit |
The components in different batches of kits cannot be used interchangeably.
【Storage conditions and validity】
The test card should be stored at 4℃~30℃, dry, dark and no freezing. It should be stored in sealed aluminum foil bag and valid for 12 months. The test card should be returned to room temperature (15℃~30℃) before opening. It should be used within 15 minutes after unsealing under the environment of 15℃~30℃ and 20% ~ 90% relative humidity.
The production date, batch number and expiration date are shown in the outer package of the product.
【Applicable instruments】
NIR-1000 dry fluoroimmunoassay analyser produced by WWHS Biotech. Inc.
【Sample requirements】
【Procedure】
【Reference interval】
By measuring 268 healthy people aged between 19 and 79, statistical analysis shows that the reference interval is less than 500 ng/ml. According to the characteristics of the local population, the reference intervals of the laboratories were established.
【Interpretation of results】
【Limitations of methods】
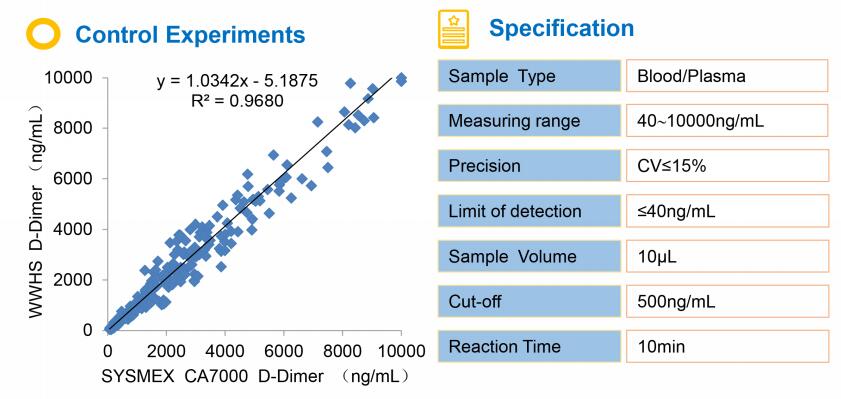
【Performance】
1. Analysis sensitivity,No more than 40ng/ml.
2. Accuracy,The relative deviation from the target value is within ±15%.
3. Precision,The within and between assay coefficient of variations are within 15%.
4. Linear range,Within the linear range (40 ~ 10000ng/ml), the linear correlation coefficient R≥0.990.
【Note】
1. This kit is only used for in vitro diagnosis.
2. The test card and sample diluent are disposable and cannot be reused.
3. Please check the integrity and validity of the kit package before use, and then open the package. When it is stored at low temperature, it should be restored to room temperature (15℃~30℃) before opening the package for use. The reagents with damaged inner package and beyond the validity period cannot be used.
4. The requirements of specimen collection and storage should be strictly observed. If the specimen is turbid, it should be centrifuged and discarded before use.
5. The used kits should be treated as potential infectious substances, and all samples, reagents and potential pollutants should be disinfected and treated according to the relevant local regulations.








Precautions
1. The kit can be used for in vitro diagnosis only.
2. Test card and buffer solution are single-use and they cannot be
reused.
3. Please inspect packaging integrity and validity of kit before
use and then unpack the product. If the product is stored at low
temperature, restore to room temperature (15℃-30℃) before unpacking
and use. Reagent cannot be used if packaging is damaged and the
validity period expires.
4. Take the test card out of the aluminum foil bag and carry out
experiment in 15min. Do not place it in the air for a long time to
avoid dampness.
5. It is required to strictly comply with the requirements for
sample collection and storage. If the sample is turbid, please
centrifuge and precipitate it before use.
6. The kit used should be disposed of as latent infective material,
and all samples, reagents and latent contaminants should be
disinfected and disposed of according to relevant local
regulations.
7. Too high or too low hematocrit of red cells may affect whole
blood test result, so verification should be conducted using other
methods.
More options, Click the following link if any interest
Respiratory & Digestive Disease Test
Sexually Transmitted Diseases Test
FAQ
1. What is fluorescent immunoassay analyzer?
The Biopanda Fluorescence Immunoassay Analyser is used by small
labs for the detection of a range of biomarkers to assist with the
diagnosis and monitoring of several medical conditions including
cardiovascular disease, inflammation, kidney disease, thyroid
conditions, and fertility.
2. Do you have CE certificate for immunoassay analyzer?
WBC analyzer belongs to IVD other/general, no need CE, Ec
declaration is enough.
3. How does fluorescence immunoassay work?
Fluorescent Immunoassays are simply a different type of
immunoassay. ... A modern fluorescent based immunoassay uses as the
detection reagent a fluorescent compound which absorbs light or
energy (excitation energy) at a specific wavelength and then emits
light or energy at a different wavelength.
4. What is indirect immunofluorescence assay?
Indirect immunofluorescence, or secondary immunofluorescence, is a
technique used in laboratories to detect circulating autoantibodies
in patient serum. It is used to diagnose autoimmune blistering
diseases.
5. Is immunofluorescence an immunoassay?
Immunofluorescence assay (IFA) is a standard virologic technique to
identify the presence of antibodies by their specific ability to
react with viral antigens expressed in infected cells; bound
antibodies are visualized by incubation with fluorescently labeled
antihuman antibody.