WWHS Biotech.Inc(exclusive marketed by Dawin) |
|
Verified Suppliers
|
|
【Product Name】
Anti-Mullerian Hormone (AMH) Rapid Quantitative Test (Fluorescence immunoassay)
【Packing Specification】
25 Tests/kit
【Intended Use】
The kit is used for quantitative determination of AMH in human whole blood,serum or plasma. It is mainly used to evaluate ovarian reserve and assist in the diagnosis of polycystic ovary syndrome .
【Test principle】
The Diagnostic Kit for AMH is a one-step chromatographic sandwich immunoassay designed for the quantitative measurement of AMH. The AMH antigen in the sample was first bound with the conjugated compound of fluorescent labeled AMH monoclonal antibody, then moved and combined with another AMH monoclonal antibody fixed on the nitrocellulose membrane, and the double antibody sandwich complex was formed at the detection line of the cellulose nitrate membrane. The quantitative detection results were obtained by NIR-1000 dry fluoroimmunoassay analyser.
【Components】
| Name | Quantity | Component |
| Test cards | 25 | The product consists of fluorescent pat (coated with fluorescently-labeled AMH antibody), nitrocellulose membrane (coated with AMH antibody and Goat anti mouse IgG antibody), absorbent paper and PVC soleplate. |
| Sample diluent | 25 (0.3mL/ tube) | Phosphate buffer |
| ID card | 1 | With specific stand curve file |
The components in different batches of kits cannot be used interchangeably.
【Storage Conditions and Validity】
The kit should be stored at 4℃~30℃, out of direct sunlight. It is valid for 18 months. The test card should be used within 15 minutes after unsealing under the environment of 15℃~30℃ and 20% ~ 90% relative humidity.
The production date, batch number and expiration date are shown in the outer package of the product.
【Applicable Instrument】
NIR-1000 dry fluoroimmunoassay analyser produced by WWHS Biotech. Inc.
【Sample Requirements】
【Test procedure】
【Reference Interval】
Test and analyze the AMH from healthy people serum, and use the 95 percentile method to determine the AMH reference interval.
| Sex | Age | Reference Interval (ng/mL) |
| Adult male | ~ | 0.92~13.89 |
| Female | 20~29 | 0.88~10.35 |
| 30~39 | 0.31~7.86 | |
| 40~50 | ≤5.07 |
It is strongly recommended that each laboratory should determine its own normal and abnormal values.
【Interpretation of test results】
【Limitation of test method】
3. The triglyceride content in the sample does not exceed 15mg/mL, the hemoglobin content does not exceed 10mg/mL, the bilirubin content does not exceed 0.5mg/mL, the cholesterol does not exceed 10mg/mL, and the relative deviation of the measurement results does not exceed ±15.0%.
4. When AMH concentration of samples reaches 160.00ng/mL, there is no hook effect.
6. When RF concentration of samples is less than 2000IU/mL, relative deviation of test result is limited to ±10.0%.
7. For samples exceeding the linearity range, test cannot be conducted after dilution.
8. Inhibin A (≤100ng/mL), Activin A (≤100ng/mL), LH (≤500mIU/mL), FSH (≤500mIU/mL) several commonly used drugs in the sample (Cefoxitin ≤2500mg/ L. Metformin≤2000mg/L, ibuprofen≤500mg/L, rifampicin≤60mg/L, doxycycline≤50mg/L), the relative deviation of the measurement results does not exceed ±15.0%
【Performance】
1. Limits of detection
No higher than 0.10ng/mL
2. Accuracy
The relative deviation to the target value is limited to ±15.0%.
The within and between assay coefficient of variations are within 15%.
Within the linear range (0.10 ~ 16.00) ng/mL, the linear correlation coefficient R≥0.990.
【Note】
1. The kit can be used for in vitro diagnosis only.
2. Test card and buffer solution are single-use and they cannot be reused.
3. Please check the integrity and validity of the kit package before use, and then open the package. When it is stored at low temperature, it should be restored to room temperature (15℃ ~ 30℃) before opening the package for use. The reagents with damaged inner package and beyond the validity period cannot be used.
4. Take the test card out of the aluminum foil bag and carry out experiment in 15min. Do not place it in the air for a long time to avoid dampness.
5. It is required to strictly comply with the requirements for sample collection and storage. If the sample is turbid, please centrifuge and precipitate it before use.
The kit used should be disposed of as latent infective material, and all samples, reagents and latent contaminants should be disinfected and disposed of according to relevant local regulations.

Other WWHS Assay Items
| Cardiac | ||||||
| cat#. | Product item | Specimen | Reaction Time | Measure Range | Clinical Range | Itended Use |
| 1 | cTnI | WB/Serum/Plasma | 12min. | 0.1-40ng/ml | <0.3ng/ml | several heart diseases including myocardial infarction and heart failure. |
| 2 | Myo | WB/Serum/Plasma | 12min. | 5-400ng/ml | <58ng/ml | acute myocardial infarction (AMI) in early stage. |
| 3 | CK-MB | WB/Serum/Plasma | 12min. | 1-200ng/ml | <5ng/ml | acute myocardial infarction (AMI) in early stage. |
| 4 | NT-proBNP | WB/Serum/Plasma | 10min. | 20-35000pg/ml | Under 75:0~347pg/mL, Over 75:0~449pg/mL | heart failure . |
| 5 | D-Dimer | WB/Plasma | 10min. | 40-10000ng/ml | <500ng/ml | disseminated intravascular coagulation (DIC),deep vein thrombosis (DVT),pulmonary embolism (PE), myocardial infarction, cerebral infarction, etc. |
| 6 | cTnI+Myo+CKMB | WB/Serum/Plasma | 12min. | same with single item | same with single item | Triple marker of myocardial infarction. |
| 7 | ST2 | WB/Serum/Plasma | 10min. | 10-400ng/ml | <35ng/ml | heart failure . |
| 8 | Lp-PLA2 | WB/Serum/Plasma | 10min. | 10-900ng/ml | <175ng/ml | Risk evaluation of ACS and atherosclerotic ischemic stroke patients. |
| 9 | S100-β | WB/Serum/Plasma | 10min. | 0.05-10ng/ml | <0.2ng/ml | Cerebral infarction, cerebral injury. |
| Inflammation | ||||||
| 10 | CRP / hs-CRP | WB/Serum/Plasma | 3min. | 0.5-200mg/L | CRP<10mg/L,hs-CRP<1mg/L | nonspecficity inflammatory marker. |
| 11 | SAA | Serum | 5min. | 1-200mg/L | <10mg/L | inflammation&infection. |
| 12 | PCT | WB/Serum/Plasma | 10min. | 0.2-100ng/ml | <0.5ng/ml | Sepsis |
| 13 | CRP+SAA | WB/Serum/Plasma | 5min. | same with single item | same with single item | inflammation&infection. |
| 14 | IL-6 | WB/Serum/Plasma | 10min. | 5-4000pg/ml | 10pg/ml | diabetes,rheumatoid arthritis,etc |
| Thyroid Hormone | ||||||
| 15 | TSH | Serum/Plasma | 15min. | 0.3-100mU/L | 0.35-5mU/L | hyperthyroidism and hypothyroidism |
| 16 | TT3 | Serum/Plasma | 15min. | 0.5-10nmol/L | 1.3-3.1nmol/L | thyroid dysfunction |
| 17 | TT4 | Serum/Plasma | 15min. | 5-300nmol/L | 66-181nmol/L | thyroid dysfunction |
| 18 | FT3 | Serum/Plasma | 15min. | 1-100pmol/L | 4-10pmol/L | thyroid dysfunction |
| 19 | FT4 | Serum/Plasma | 15min. | 5-300pmol/L | 19-39pmol/L | thyroid dysfunction |
| Tumor Marker | ||||||
| 20 | AFP | Serum/Plasma | 15min. | 2.5-200ng/ml | <20ng/ml | pregnancy cancer |
| 21 | CEA | Serum/Plasma | 15min. | 1-200ng/ml | <5ng/ml | colon cancer, colorectal cancer,etc. |
| 22 | NSE | Serum/Plasma | 15min. | 1-400ng/ml | <16ng/ml | non-small cell lung cancer |
| 23 | FOB | fecal specimens | 10min. | 50-1000ng/ml | <100ng/ml | Abnormal recessive gastrointestinal bleeding |
| 24 | PG II | Serum/Plasma | 15min. | 1-100ug/L | PGI/PGII>3.0 | gastric abnormalities |
| 25 | PG I | Serum/Plasma | 15min. | 2.5-200ug/L | >70ng/ml | gastric abnormalities |
| 26 | TPSA | Serum/Plasma | 15min. | 0.5-40ng/ml | <4ng/ml | prostate cancer |
| 27 | FPSA | Serum/Plasma | 15min. | 0.1-10ng/ml | <1ng/ml | prostate cancer |
| 28 | CA12-5 | Serum/Plasma | 15min. | 20-500U/ml | <35U/ml | ovarian cancer |
| 29 | CA15-3 | Serum/Plasma | 15min. | 10-400U/ml | < 25 U/mL | breast cancer |
| 30 | HE4 | Serum/Plasma | 15min. | 50-2000pmol/L | <140 pmol/L | ovarian cancer |
| 31 | CA19-9 | Serum/Plasma | 15min. | 10-400U/ml | < 27 U/mL | pancreatic cancer |
| 32 | β-HCG | Serum/Plasma | 15min. | 5-400mIU/ml | <10 mIU/mL | Early pregrancy, ectopic HCG cancer,incomplete abortion |
| 33 | CK19(Cyfra21-1) | Serum/Plasma | 15min. | 0.5-50ng/ml | <2.5ng/ml | non-small cell lung cancer |
| Fertility | ||||||
| 34 | HCG / β-HCG | Serum/Plasma | 10min. | 5-20000mIU/ml | <5 mIU/mL | early pregrancy. |
| 35 | AMH | Serum/Plasma | 10min. | 0.1-16ng/ml | Male: 20-60 years old, 0.92-13.89 ng/mL Female: 20-29 years old, 0.88-10.35 ng/mL 30-39 years old, 0.31-7.86 ng/mL 40 -50 years old, <5. 07 ng/mL | ovarian reserve level |
| Gastrointestinal | ||||||
| 36 | FOB | fecal specimens | 10min. | qualitative | qualitative | gastrointestinal hemorrhage. |
| 37 | TRF | fecal specimens | 10min. | qualitative | qualitative | gastrointestinal hemorrhage. |
| 38 | FOB+TRF | fecal specimens | 10min. | qualitative | qualitative | gastrointestinal hemorrhage. |
| Infection | ||||||
| 39 | C.Pneumonia | WB/Serum/Plasma | 15min. | qualitative | qualitative | CP infection |
| 40 | M.Pneumonia | WB/Serum/Plasma | 15min. | qualitative | qualitative | MP infection |
| 41 | Covid-19 Antigen | nasal swab, throat swab or nasal wash/aspirate specimens | 15min. | qualitative | qualitative | respiratory tract |
| 42 | Covid-19 Ab IgG/IgM | WB/Serum/Plasma | 15min. | qualitative | qualitative | respiratory tract |
| 43 | FluA | nasal swab, throat swab or nasal wash/aspirate specimens | 15min. | qualitative | qualitative | respiratory tract |
| 44 | FluB | nasal swab, throat swab or nasal wash/aspirate specimens | 15min. | qualitative | qualitative | respiratory tract |
| Renal injury | ||||||
| 45 | CysC | WB/Serum/Plasma | 5min. | 0.4-9mg/L | 0.5-1.1mg/L | renal function |
| 46 | NGAL | Urine | 10min. | 10-1500ng/mL | <132ng/mL | acute kidney injury. |




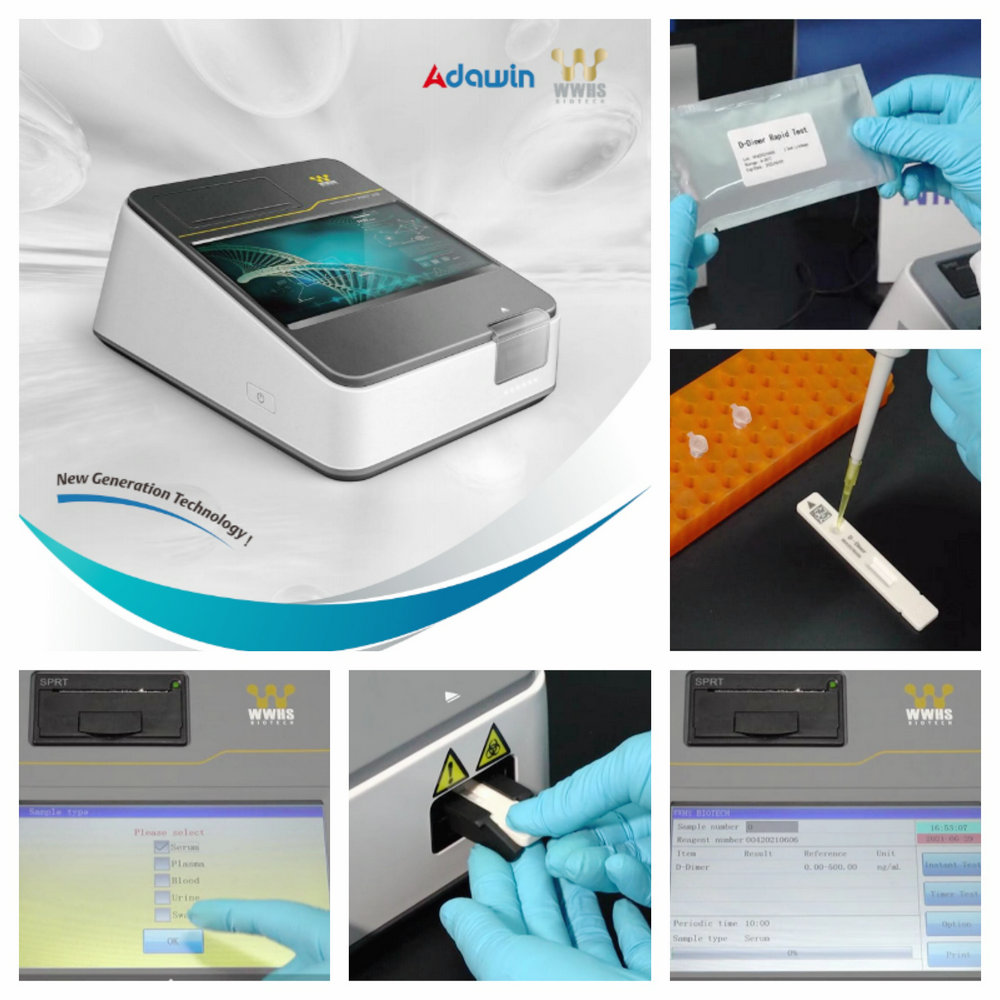


Test Method
1. Please thoroughly read the specification before test. Frozen
test card and sample should be placed at room temperature (15-30) ℃
for at least 30min before use.
2. Start NIR-1000 dry fluoroimmunoassay analyser and verify quality
control according to the specification.
3. The quality of reagent is controlled using internal quality
control product (not provided) of the company and result should be
controllable.
4. Take out the test card from the aluminum foil bag and use it
within 15min
5. Place the test card on a clean horizontal table top and label
it.
6. Take 10μL of sample and add it into HbA1c sample buffer
(1.00mL). Then, mix the solution evenly, take 100μL of the solution
and add it into the well.
7. Insert the test card into NIR-1000 dry fluoroimmunoassay
analyser, keep time for 10min and press 【Instant test】. The
analyser will judge and read the test result automatically. Or
press 【Fixed time test】 to keep time for 10min automatically. The
analyser will judge and read the test result automatically and
display it in the screen.
Applicable Instrument
WWHS NIR-1000 dry fluoroimmunoassay analyser
Advantage
Immunofluorescence technology
Testing time less than 15 minutes
Easy-to-use touch-screen platform,multi-language system
FAQ
Q: What's the minimum order quantity (MOQ)?
A: For most of our medical products, even order for only one unit
is warmly welcomed.
Q: Can you do OEM/ private label?
A: Of course we can do OEM/private label for you.
Q: How about your delivery time?
A: 2~10 day depends your order quantity.
Q: What is your terms of delivery?
A: DHL, UPS, FedEx, TNT, EMS, customer's freight forwarder is also available.
Q: What is your terms of payment?
A: Trade Assurance, T/T in advance, L/C, Western union, paypal
Please suggest your preferred payment method.
Q: Do you have certification?
A: ISO13485, CE
Q: Do you test all your goods before delivery?
A: Yes, we have 100% test before delivery
Q:How about after-sale serive ?
A:We will send you any parts within 15 months warranty, and supply
you technology support for long time, solve problem as soon as
possible .