CITEST DIAGNOSTICS INC. |
|
Verified Suppliers
|
|
COC/AINE(COC) Drug Abuse Diagnosis Fast Reading Oral Fluid Test Kits Convenient And High Sensitivity Accurate CE
| Product features | Parameters |
| Principle | Chromatographic Immunoassay |
| Format | Dipstick, Cassette, Panel, Device, Cup |
| Specimen | Urine,Oral Fluid,Powder,WB/S/P,Hair |
| Certificate | CE/FDA |
| Reading Time | 5 minutes |
| Pack | 10T/25T/40T/50T |
| Storage Temperature | 2-30°C |
| Shelf Life | 2 Years |
| Sensitivity | 97.80% |
| Accuracy | 98.00% |
| Cut-Off | 100 ng/mL, 150 ng/mL, 300 ng/mL*,20 ng/mL* ,50 ng/mL,0.5 ng/mg |
Application And Description:
How to use?

DIRECTIONS FOR DEVICE USE
Allow the test device, specimen, and/or controls to reach room
temperature (15-30ºC) prior to testing. Instruct the donor to not
place anything in the mouth including food, drink, gum or tobacco
products for at least 10 minutes prior to collection.
1. Remove the test device from the sealed pouch and use it within
one hour.
2. Take off the device cap and insert the absorbent wick to the
mouth, put it under the tongue to collect oral fluid until the
control line appears.
3. Put the device cap back outside of the absorbent wick and lace
the test device on a clean and level surface. See illustration
below.
4. Read results at 10 minutes. Do not read results after 1 hour.
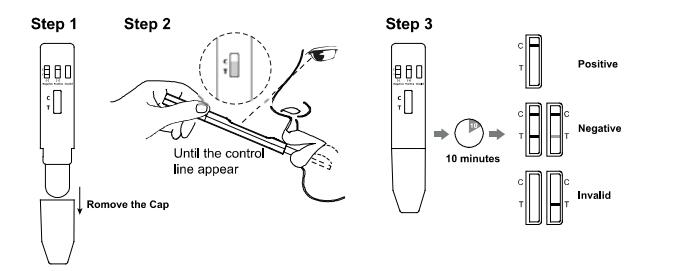
INTERPRETATION OF RESULTS
(Please refer to the illustration above)
NEGATIVE:* Two lines appear. One colored line should be in the control line region (C), and
another apparent colored line should be in the test line region
(T). A negative result indicates that the Synthetic Marijuana
metabolite concentration is below the detectable level (25ng/mL).
*NOTE: The shade of color in the test line region (T) may vary, but it
should be considered negative whenever there is even a faint
colored line.
POSITIVE: One colored line appears in the control line region (C). No line appears in the test line region (T). A positive
result indicates that the COC/AINE(COC) concentration exceeds
the detectable level (20 ng/ml).
INVALID: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are
the most likely reasons for control line failure. Review the
procedure and repeat the test using a new test. If the problem
persists, discontinue using the lot immediately and contact your
local distributor.
Order Information
| Cat. No. | Product | Specimen | Pack |
| DCO-102 | Coc/aine (COC) Rapid Test Cassette | Urine | 40T |
| DCO-114 | Coc/aine (COC) Rapid Test Panel | Urine | 40 T |
| DCO-101 | Coc/aine (COC) Rapid Test Dipstick | Urine | 50 T |
| DCO-802 | Coc/aine (COC) Rapid Test Cassette | Oral Fluid | 25 T |
| DCO-803 | Coc/aine (COC) Rapid Test Device | Oral Fluid | 25 T |
| DCO-402 | Coc/aine (COC) Rapid Test Cassette | WB/S/P | 40 T |
| DCO-X14 | Coc/aine (COC) Rapid Test Panel | Powder | 25 T |
| DCO-H902 | Coc/aine (COC) Rapid Test Cassette | Hair | 10 T |