PURIFA Medical Production Co.,Ltd |
|
Verified Suppliers
|
|
Packed with Easy-to-draw Polybag in High quality Box
50 Pcs /Box , 2500Pcs / Carton, LWH: 52CM X 42CM X 42CM


BFE ≥ 98% or Above Filtration Efficiency; In conformity with EU Standard;

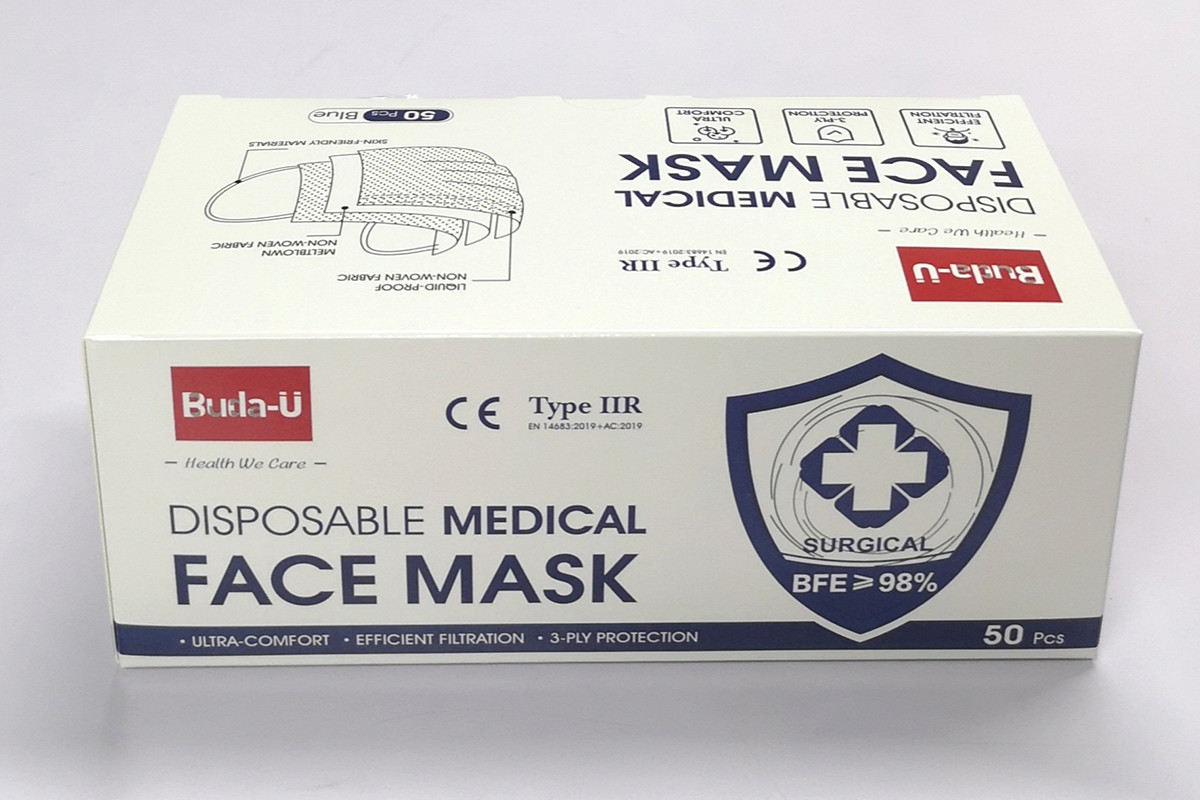
Easy-To-Draw Polybag, High Quality Packing Box; 50 Pcs / Box


Manufacturing Date and Expiration Date, EC REP Printed on the Box; High Breathability ;


Meeting All Requirements of EN 14683: 2019 + AC:2019; DOC
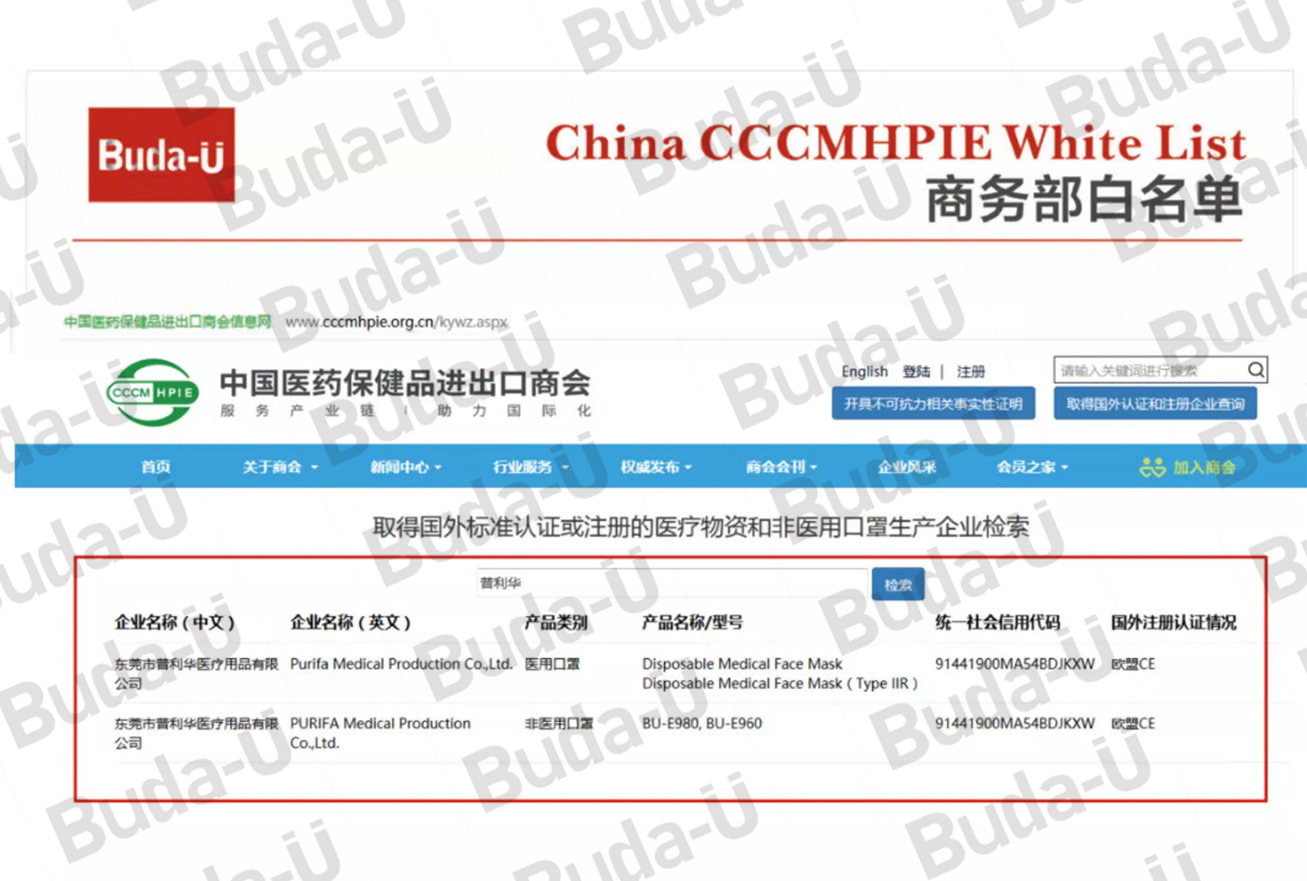
China CCCMHPIE White List
Frequently Asked Questions :
Question:
We have Heard Quite Much About Type I, Type II, and Type IIR Face Mask. What Are Exactly Type I , Type II, Type IIR About ?
Answer:
The following Table can indicate clearly on the performance requirements of Type I, Type II and Type IIR. Type II can be used for medical settings, Type IIR Face Masks are used as surgical environments since it's Splash resistance has mandatory requirements.
Table — Performance Requirements For Medical Face Masks (EN 14683:2019 +AC:2019)
| Test | Type I a | Type II | Type IIR |
| Bacterial filtration efficiency (BFE), (%) | ≥ 95 | ≥ 98 | ≥ 98 |
Differential pressure (Pa/cm2) |
< 40 |
< 40 |
< 60 |
| Splash resistance pressure (kPa) | Not required | Not required | ≥ 16.0 |
| Microbial cleanliness (cfu/g) | ≤ 30 | ≤ 30 | ≤ 30 |
| a Type I medical face masks should only be used for patients and other persons to reduce the risk of spread of infections particularly in epidemic or pandemic situations. Type I masks are not intended for use by healthcare professionals in an operating room or in other medical settings with similar requirements. | |||
To Get More Information About the EU Medical Face Masks Standard
Download EU Medical Face Masks Standard : EN 14683 : 2019 + AC : 2019
Face Mask, Disposable Face Mask,disposable mask ,3PLY mask,3ply face mask,Medical Face Mask,Medical mask, Dust mask,EN14683 Mask,Medical device, ASTM Face Mask, ASTM Surgical Mask