Labnovation Technologies, Inc. |
|
SARS-CoV-2 Antigen Rapid Test Kit Nasal Swab Virus Antigen Rapid Diagnostic CE Certificated Simple Operation
Intend Use
This rapid test kit is intended for the qualitative detection of SARS-CoV-2 viral nucleocapsid antigens from human anterior nasal of secretion from individuals suspected of COVID-19.

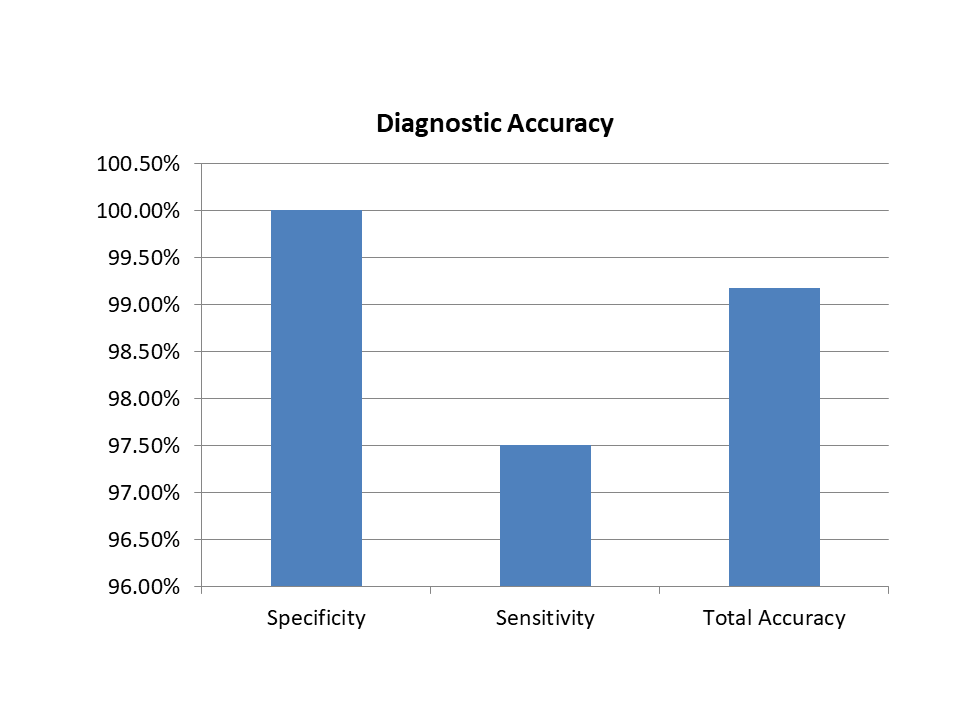
Diagnostic Accuracy
| Name | Sensitivity | Specificity | Total Accuracy |
SARS-CoV-2 Antgen Rapid Test Kit | 97.50% | 100.00% | 99.17% |
Product Feature

Main Components

Use Step
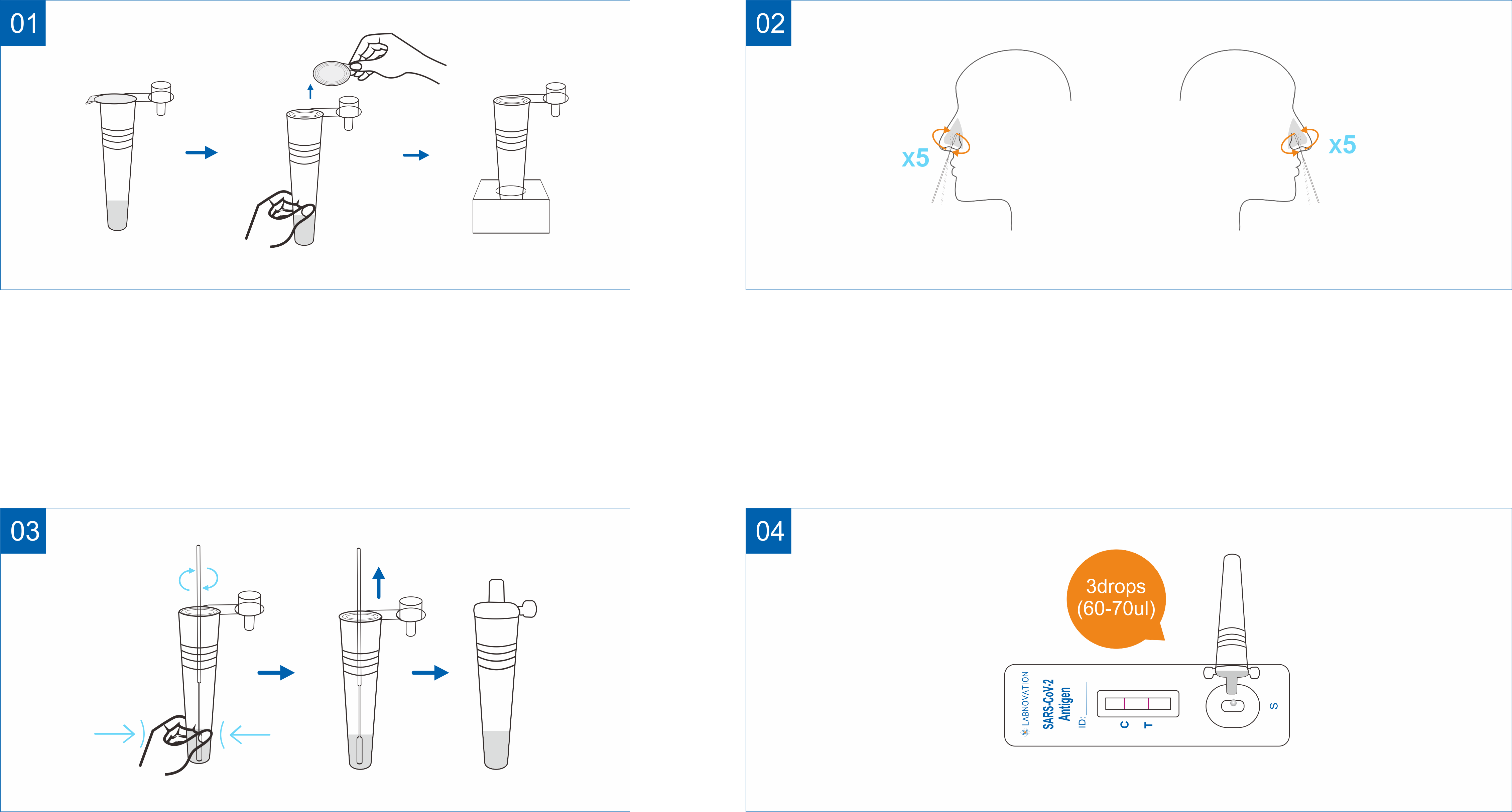
Please check the expiration date printed on the BOX. Do not use it beyond the expiration date. Lay all the supplied materials on a clean, dry and flat surface.
Take sample tube with sample extraction buffer and remove the sealed film of the sample tube. Then place the tube in the tube stand (The tube stand is the outer box, see below.)
Open the sealed pouch and remove the test cassette. Lay it face up on a clean, dry and flat surface.
Remove the swab from the container, being careful NOT to touch the soft end, which is the absorbent tip.
Gently, insert the entire absorbent tip of the swab (around 1.5 cm) into your nostril. Slowly, rotate the swab in a circular against the inside walls of your nostril 5 times or more. Be sure to collect any nasal drainage that maybe present on the swab. Gently remove the swab. Use the same swab to repeat steps in the other nostril and slowly, take out the swab.
Insert the swab into the sample tube with prefilled extraction buffer. Mix well and squeeze the swab 10-15 times by compressing the walls of the tube against the swab. Roll the swab head against the inner wall of the tubes as you remove it. Try to release as much liquid as possible. Dispose of the used swab in accordance with applicable local regulations.
Close the cap of the sample tube. Add 3 full drops of the mixed solution vertically into the sample well (S) of the test cassette.
Read the result 15-20 minutes after adding the sample. Result got after 20 minutes is invalid.
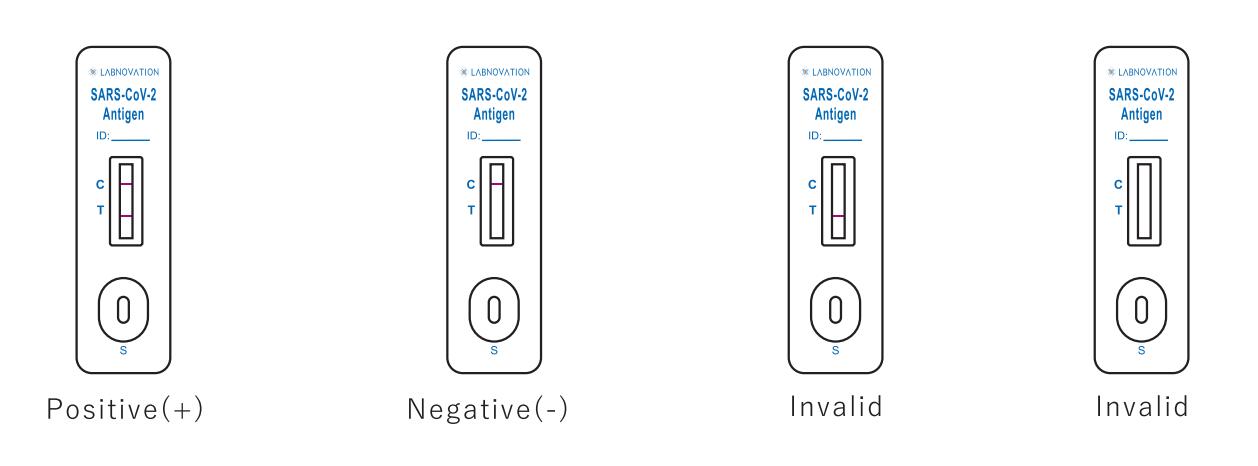
Result Interpretation
POSITIVE: Two colored bands appear on the membrane. One band appears in the control region (C) and another band appears in the test region (T).
NEGATIVE: Only one colored band appears, in the control region (C). No apparent colored band appears in the test region (T).
INVALID: If there is no Control line (C) or only a Test line (T) in the result window, the test did not run correctly and the results are not valid.
Virus Sources
| Global high frequency mutation | Alpha / B.1.1.7(U.K.) | Beta I B.1.351(South Africa) |
| Gemma I P.1(Brazil) | Kappa I B.1.617.1(India) | Delta I B.1.617.2(India) |
| C.37,ect | Alpha I B.1.17(U.K.) | B.1.36.16.etc |
| A.2.5,etc | A.23.1 | Alpha I B.1.17(U.K.) |
| B.1.1.33.etc | C.1.1.etc. | others |
Applicable
Regular home testing
Individuals who frequently work in high-risk environments or who do not have a fixed place of work
Busy office workers who want to check themselves in a short time in order to feel at ease
Other Information
Certificate
