Labnovation Technologies, Inc. |
|
Rapid Antigen Test Kit SARS-CoV-2 Antigen Self Testing 5 Tests High Specificity Antigen Rapid Test Kit
Intend Use
This rapid test kit is intended for the qualitative detection of SARS-CoV-2 viral nucleocapsid antigens from human anterior nasal of secretion. Testing result of the antigen test can be used for early isolation of patients with suspected infection, but it cannot be used as diagnosis basis of SARS-CoV-2 infection. Further nucleic acid detection should be carried out for suspected population whose antigen test result is positive or negative.
Product Details
| Item | Value |
| Model Number | LX-401305 |
| Package | 5 Tests/Kit |
| Warranty | 24 Months |
| Quality Certification | CE, MSDS |
| Safty Standard | ISO13485 |
| Sample Type | Nasal Swab |
| Sample volume | 3 Full drops |
| Test Speed | Within 15 minutes |
| Specificity | 100.00% |
| Sensitivity | 97.45% |
Product Feature
Advantage
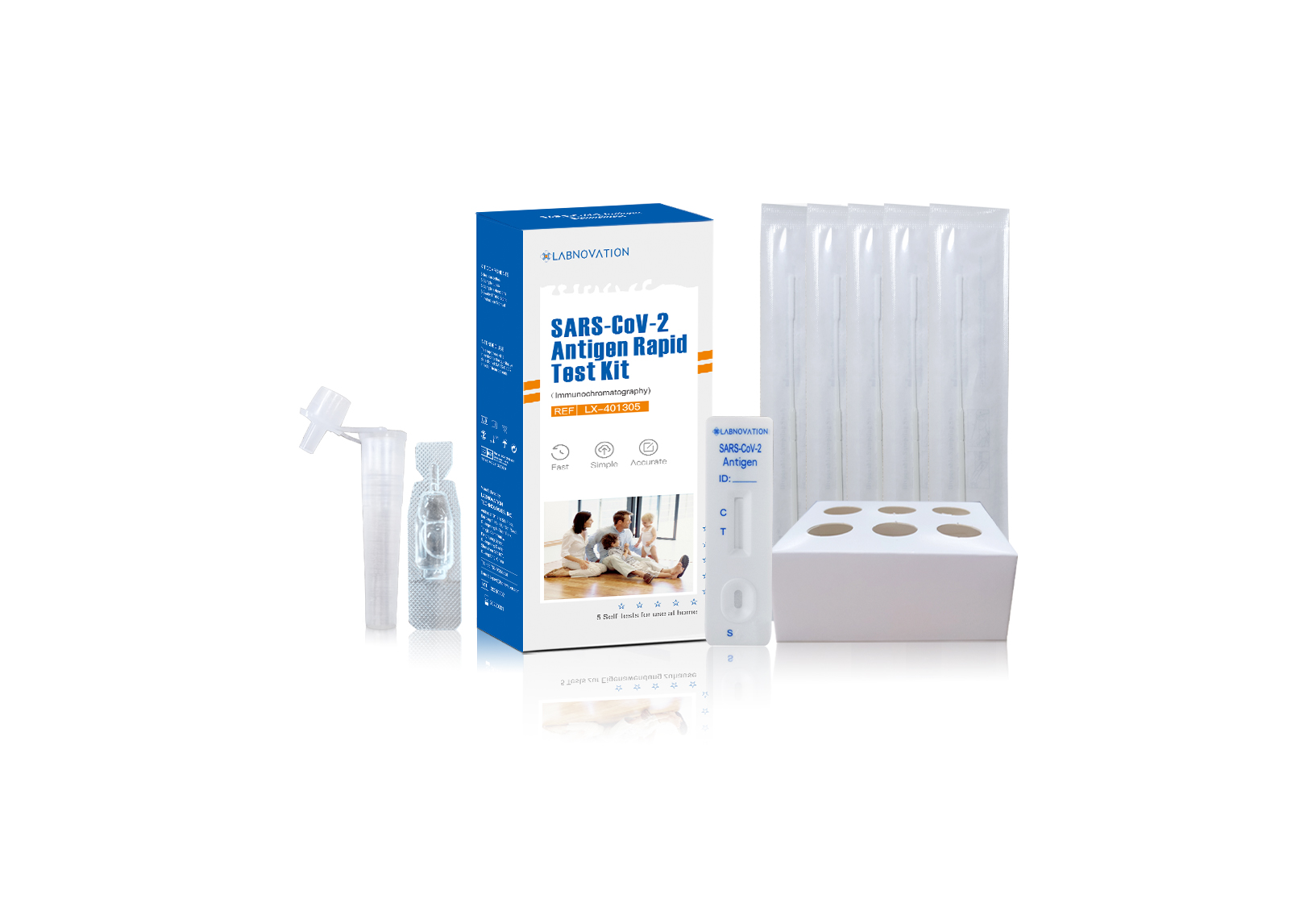

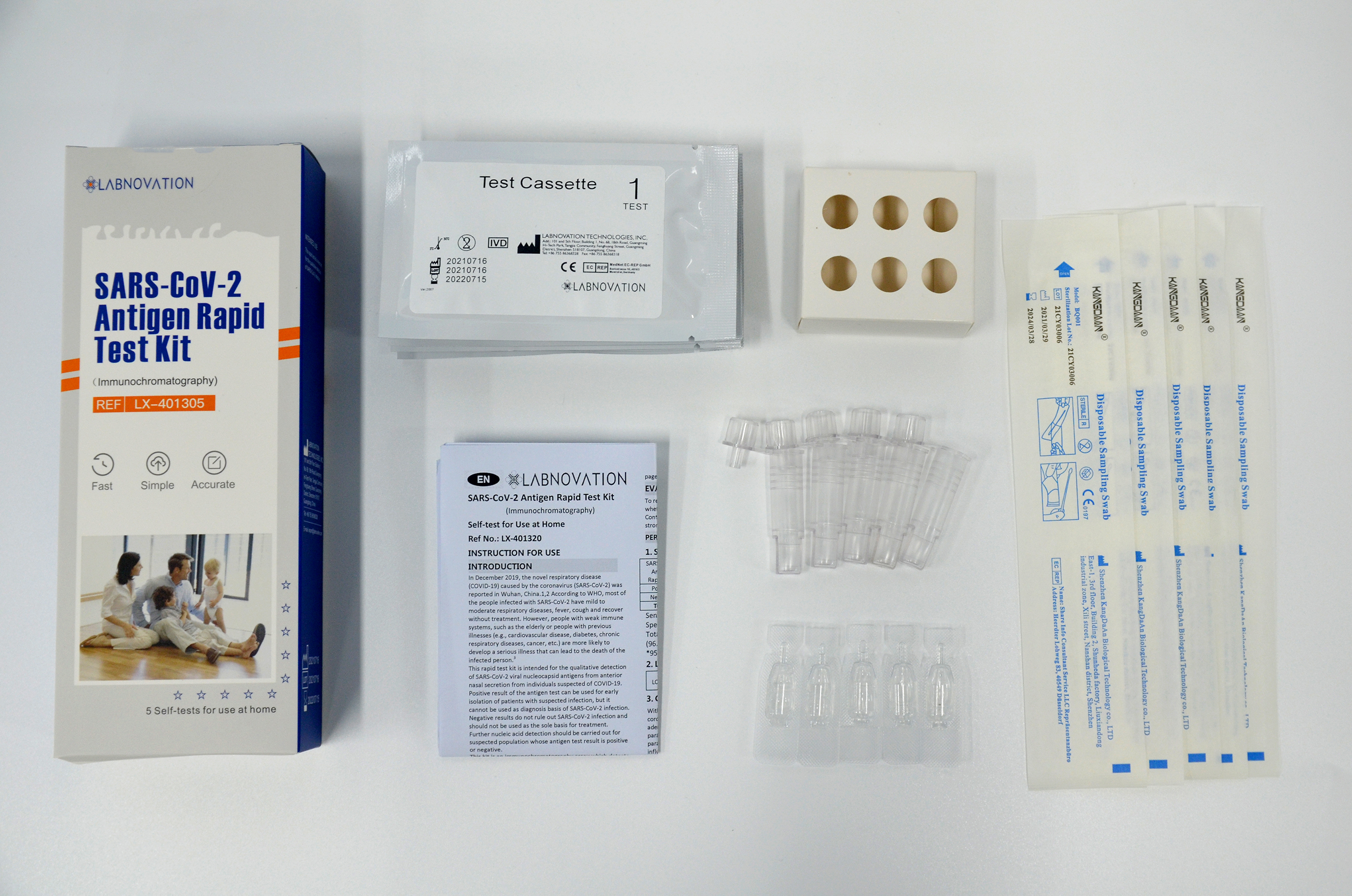
Main Components
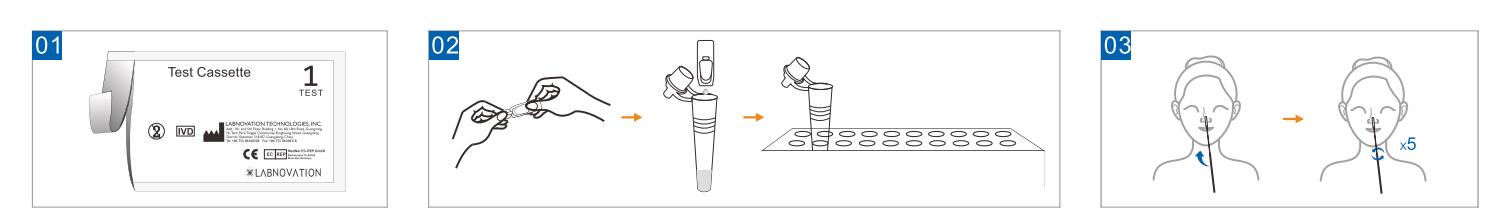
TEST PREPARATION
Let test cassette and test components stand at a room temperature (15℃ to 27℃) before performing the test.
Lay all the supplied materials on a clean, dry and flat surface.
Use Step
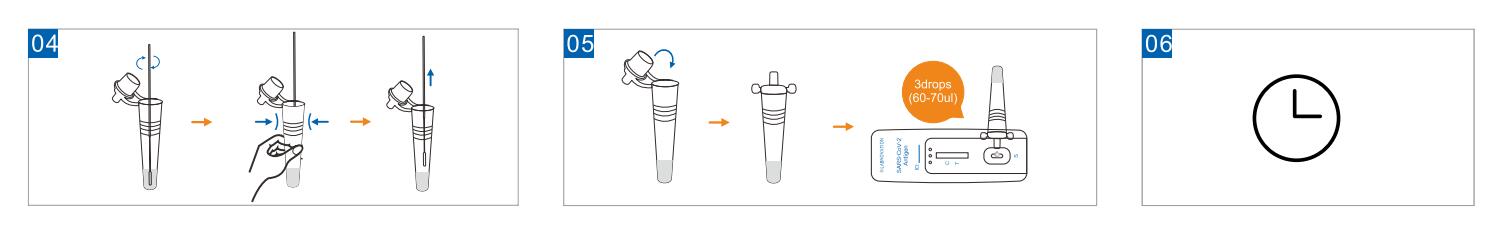
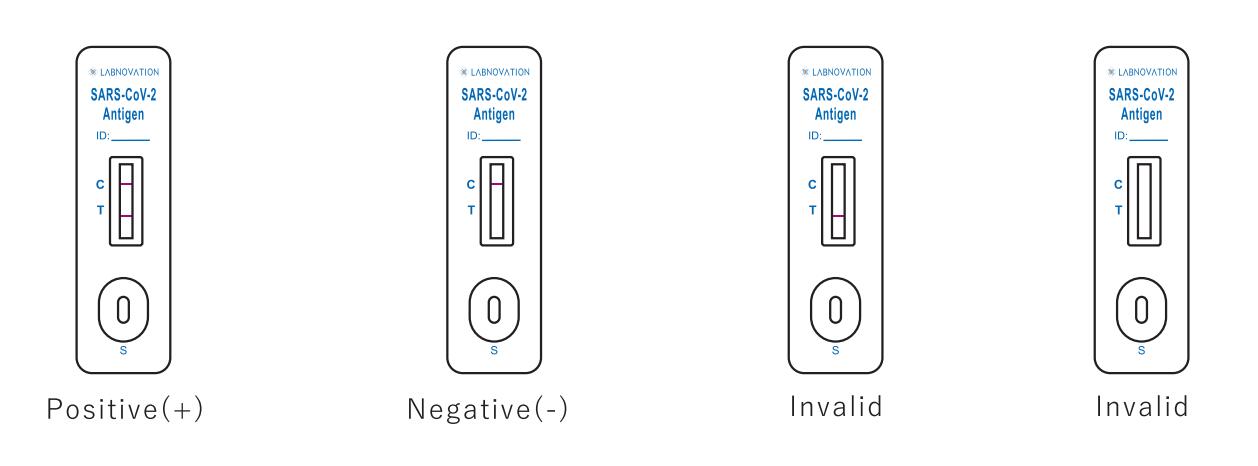
Result Interpretation
POSITIVE: Two colored bands appear on the membrane. One band appears in the control region (C) and another band appears in the test region (T).
NEGATIVE: Only one colored band appears, in the control region (C). No apparent colored band appears in the test region (T).
INVALID: If there is no Control line (C) or only a Test line (T) in the result window, the test did not run correctly and the results are not valid.
Virus Sources
| Global high frequency mutation | Alpha / B.1.1.7(U.K.) | Beta I B.1.351(South Africa) |
| Gemma I P.1(Brazil) | Kappa I B.1.617.1(India) | Delta I B.1.617.2(India) |
| C.37,ect | Alpha I B.1.17(U.K.) | B.1.36.16.etc |
| A.2.5,etc | A.23.1 | Alpha I B.1.17(U.K.) |
| B.1.1.33.etc | C.1.1.etc. |
Other Information
n
FAQ
Stock 3-7 days in general. Or please contact us by email for specific lead time base on your order quantities.
Quality is priority. We attach great importance to quality control from the very beginning to the end. All raw material we used are non-toxic, environmental-friendly. We have a stric professional QA/QC team to ensure the quality.
Yes, we accept both OEM and ODM for customers.
We can accept EXW, FOB, CIF, etc. You can choose one which is the most convenient for you.
TT, DP, DA, etc
Certificate