Labnovation Technologies, Inc. |
|
Rapid Test Kit SARS-CoV-2 Antigen & Influenza A/B rapid Test Kit High Accuracy Rapid Test Kit
Intended Use
Specification
| Item | Antigen test strip performance against PCR | Influenza A test strip performance against PCR | Influenza B test strip performance against PCR |
| Sensitivity | 98.03% | 93.30% | 97.00% |
| Specificity | 100.00% | 91.00% | 96.40% |
Main Components

Feature

Use Step
Apply 2 full drops of the treated sample (60μl-70μl) vertically into each of the two sample wells of the test cassette.
Observe the test results immediately within 15~20 minutes, the result is invalid over 20 minutes.

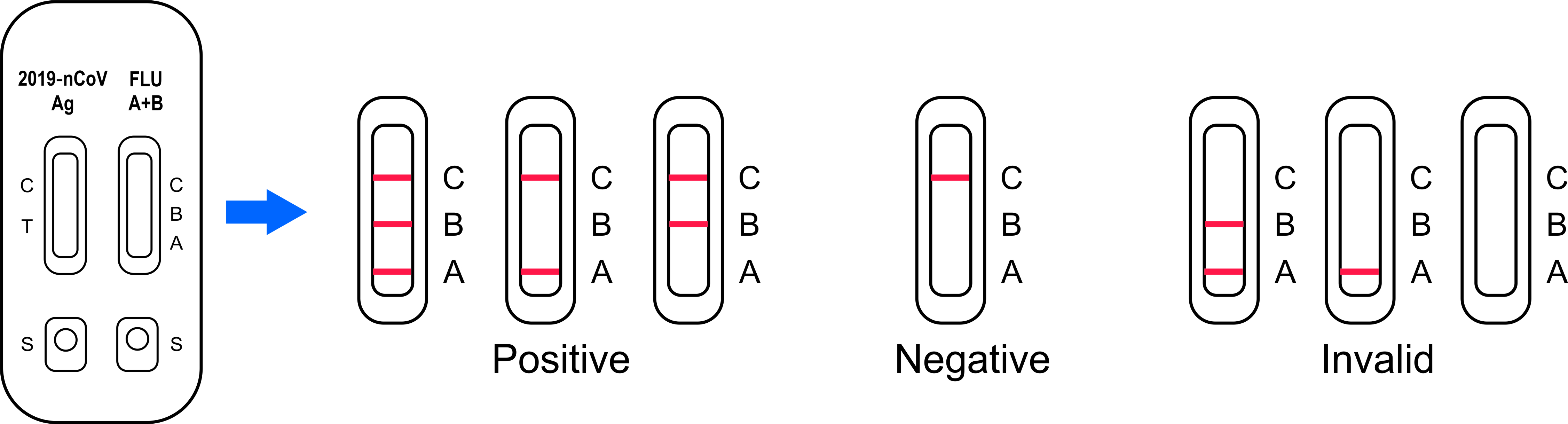
Interpretation Of Result
POSITIVE: The presence of T (T on nCoV /A or B on Flu) and C lines within the reaction window indicate a positive result on SARS-CoV-2 or Flu A and/or B infection or co-infection.
NEGATIVE: One colored line appears in the control region(C). No apparent colored line appears in the test region (T on nCoV /A or B on Flu). The negative result does not indicate the absence of analytes in the sample, it only indicates the level of tested analytes in the sample is less than the minimum detection limit
INVALID: No colored lines appear, or control line fails to appear, indicating that the operator error or reagent failure. Verify the test procedure and repeat the test with a new testing device.
Advantage