Rapid Test Kit Antigen And Antibody Combo Detection Dengue
NS1/IgG/IgM Rapid Test Kit 25 Tests For Professional Test
Intenden Use
Dengue NS1 Antigen & IgG/IgM Antibody Rapid Test Kit is a rapid
Immunochromatography for the qualitative detection of antibodies
(IgM and IgG) and dengue virus NS1 antigen to dengue virus in
serum/plasma/whole blood to aid in the diagnosis of Dengue viral
infection.
Specifications
| Brand | Labnovation |
| Name | Dengue NS1 Antigen & IgM/IgG Antibody Rapid Test Kit |
| No | LQ-000101 |
| Sample Type | Serum, Plasma, Whole Blood |
| Sample Volume | NS1:3 Full drops, IgG/IgM:1 Full Drop |
| Package Size | 25 Tests/Kit |
| Product Shelf Life | 24 Months |
| Test Speed | Within 15 minutes |
| Sample Volume | NS1:3 Full drops, IgG/IgM:1 Full Drop |
| Storage Condition | Store 2-30℃ |
| Humidity | ≤60% |
| Method | Immunochromatography |
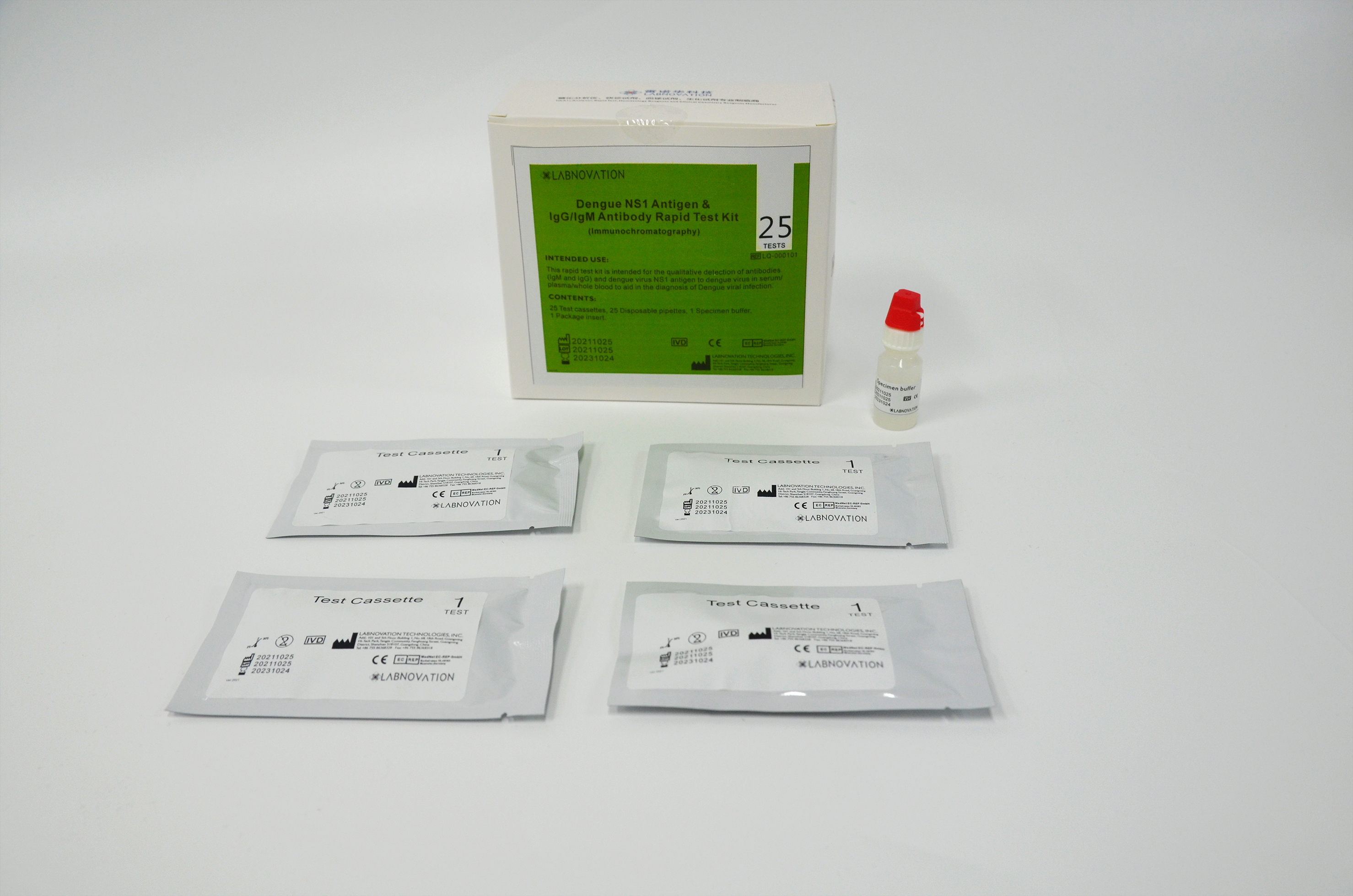
Main Components
- 25 Test Cassettes
- 25 Disposable Pipettes
- 1 Specimen Buffer
- 1 Instruction For Use
Material Needed But Not Provided
- Specimen Collection Containers
- Centrifuge (for serum/plasma sample)
- Timer
Analytical Resualts
| Item | IgG | IgM | NS1 |
| Specificity | 98.46% | 98.44% | 99.02% |
| Sensitivity | 96.19% | 95.37% | 97.92% |
| Accuracy | 97.67% | 97.33% | 98.67% |
Test Method
Instructions must be read entirely before taking the test. Allow
the test device controls to equilibrate to room temperature for 30
minutes (20℃-30℃) prior to testing. Do not open the inner packaging
until ready, it must be used in one hour if opened (Humidity≤60%,
Temp: 20℃-30℃). Please use immediately when the humidity>60%.
Use Step
- Take off the outer packing, put the panel onto the desk with the
sample window up.
- NS1: Drop 3 drops (80-100μl) of serum/plasma/whole blood vertically
into the sample well of NS1. If the whole blood sample is thick,
add about 1 drop (40-50μl) of specimen buffer into the sample well
of NS1
- IgG/IgM: Drop 1 drop (30μl) of serum/plasma/whole blood vertically into the
sample well of IgG/IgM, add about 2 drops (80-100μl) of specimen
buffer into the sample well of IgG/IgM.
- Observe the test results immediately within 15-20 minutes, the
result is invalid after 20 minutes.
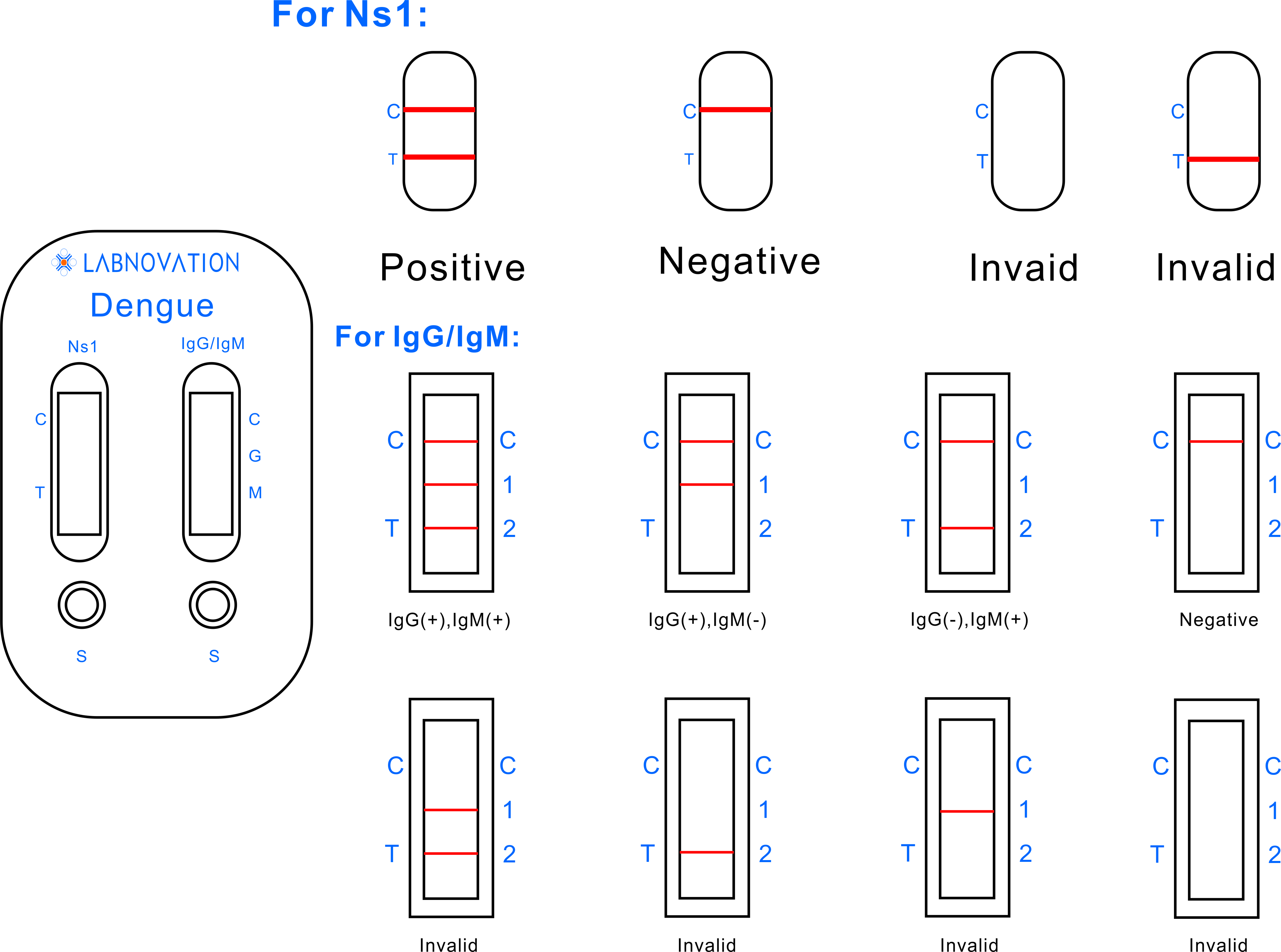
Interpreation Of Result
- POSITIVE: Two distinct red lines appear. One line should be in the control
region (C) and the other line should be in the test region (T).
- NEGATIVE: One red line appears in the control region(C). No apparent red or
pink line appears in the test region (T).
- INVALID: No red lines appear or control line fails to appear, indicating
that the operator error or reagent failure. Verify the test
procedure and repeat the test with a new testing device.
- POSITIVE: Two distinct red lines appear. One line should be in the control
region (C) and another line should be in the IgG test region (G),
indicating the Dengue IgG positive.
- POSITIVE: Two distinct red lines appear. One line should be in the control
region (C) and another line should be in the IgM test region (M),
indicating the Dengue IgM positive.
- POSITIVE: Three distinct red lines appear in the control region (C), the IgG
test region (G) and the IgM test region (M), indicating the Dengue
IgG and Dengue IgM positive.
- NEGATIVE: One red line appears in the control region(C). No apparent red or
pink line appears in the test region (G and M).
- INVALID: No red bands appear or control line fails to appear, indicating
that the operator error or reagent failure.
Precautions
- For IN VITRO diagnose only.
- Do not use after the expiration date.
- The test result is invalid after 20 minutes.
- The strength of the quality control line does not indicate the
quality problem of the reagent, a test result that is clearly
visible demonstrates the reagent is effective.
- All samples and reagents should be considered potentially hazardous
and handled in the same manner as an infectious agent after use.
- Do not use other kinds of quality control sample to test the
reagent. Components of different batches cannot be exchanged for
use to avoid erroneous results
FAQ
MOQ is 10000 pcs
We choose Air cargo or Ocean cargo.
It will depend on your order quantities. We will confirm with you
after you placed the order.
Business to business account.
- Do you support OEM/ODM order?
Of couse, we support OEM/ODM order.
- Are you a manufacturer or trading company?
We are manufacturer.
Any questions or needs you can contact us online / by e-mail, ect.
we will get back to you within 24 hours.

