Xi'an Kacise Optronics Co.,Ltd. |
|
Verified Suppliers
|
|
KFDO310 integrated on-line fluorescence dissolved oxygen sensor is designed and made based on the quenching principle of excited fluorescence of specific substances in physics. The blue light from the light-emitting diode illuminates the fluorescent material on the inner surface of the fluorescent cap. The fluorescent material on the inner surface is excited and emits red light. By detecting the phase difference between the red light and the blue light, and comparing it with the internal calibration value, the concentration of oxygen molecule can be calculated, and the final value can be output by temperature automatic compensation.
No electrolytes, no polarization
No need to consume oxygen, not affected by the flow rate
Built-in temperature sensor, automatic temperature compensation
Free from chemicals like sulfides
Small drift, fast response, more accurate measurement
Maintenance-free, long service life, lower use cost
Fluorescent caps are easy to replace
RS-485 interface, Modbus-RTU protocol
| Model number | KFDO310 |
| Measuring principle | fluorescence |
| Range | 0ー20 mg/L (0ー200% saturation, 25 °C) |
| Resolution | 0.01 mg/l, 0.1 °C |
| Precision | ± 2% f.s. , ± 0.5 °C |
| Temperature compensation | Automatic temperature compensation (PT1000) |
| Output mode | RS-485 bus, Modbus-RTU protocol |
| Working conditions | 0ー45 °C, < 0.2 mpa |
| Storage temperature | - 5 ~ 65 °C |
| Installation mode | Immersion mounting |
| Cable length | 5 meters, other length can be customized |
| Power consumption | < 0.05 W |
| Power supply | 12 ~ 24 VDC ± 10% |
| Protection level | IP68 |
| Calibration | Two-point calibration |
| Fluorescent cap life | Guaranteed Use for one year (under normal use) |
| Material for sensor housing | Pom and 316L stainless steel |
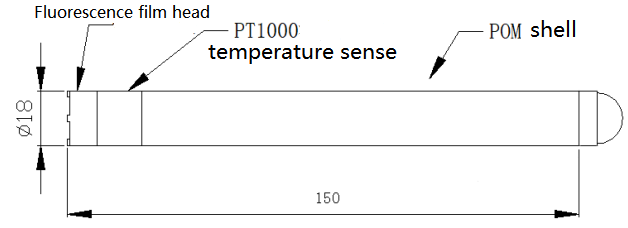
The temperature sensing part should be immersed below the liquid surface to avoid collision with the film head surface. The head part of the membrane should be free from sediment.

Henry’s law determining the dissolved oxygen concentration at 20 degrees C and 100% air saturation (1 kg water = 1 L water)
100% air saturation is the equilibrium point for gases in water. This is because gas molecules diffuse between the atmosphere and the water’s surface. According to Henry’s Law, the dissolved oxygen content of water is proportional to the percent of oxygen (partial pressure) in the air above it 13. As oxygen in the atmosphere is about 20.3%, the partial pressure of oxygen at sea level (1 atm) is 0.203 atm. Thus the amount of dissolved oxygen at 100% saturation at sea level at 20° C is 9.03 mg/L ¹⁰.
The equation shows that water will remain at 100% air saturation at equilibrium. However, there are several factors that can affect this. Aquatic respiration and decomposition lower DO concentrations, while rapid aeration and photosynthesis can contribute to supersaturation. During the process of photosynthesis, oxygen is produced as a waste product. This adds to the dissolved oxygen concentration in the water, potentially bringing it above 100% saturation ¹⁴. In addition, the equalization of water is a slow process (except in highly agitated or aerated situations). This means that dissolved oxygen levels can easily be more than 100% air saturation during the day in photosynthetically active bodies of water ¹⁴.

Dissolved oxygen often reaches over 100% air saturation due to photosynthesis activity during
the day

Supersaturation of water can be caused by rapid aeration from a dam.
Supersaturation caused by rapid aeration is often seen beside hydro-power dams and large waterfalls. Unlike small rapids and waves, the water flowing over a dam or waterfall traps and carries air with it, which is then plunged into the water. At greater depths and thus greater hydrostatic pressures, this entrained air is forced into solution, potentially raising saturation levels over 100%.
Rapid temperature changes can also create DO readings greater than 100%. As water temperature rises, oxygen solubility decreases. On a cool summer night, a lake’s temperature might be 60° F. At 100% air saturation, this lake’s dissolved oxygen levels would be at 9.66 mg/L. When the sun rises and warms up the lake to 70° F, 100% air saturation should equate to 8.68 mg/L DO. But if there is no wind to move the equilibration along, the lake will still contain that initial 9.66 mg/L DO, an air saturation of 111%.