[Product Name]PCT.pdf
Procalcitonin (PCT) Rapid Quantitative Test (Fluorescence
immunoassay)
[Intended Use]
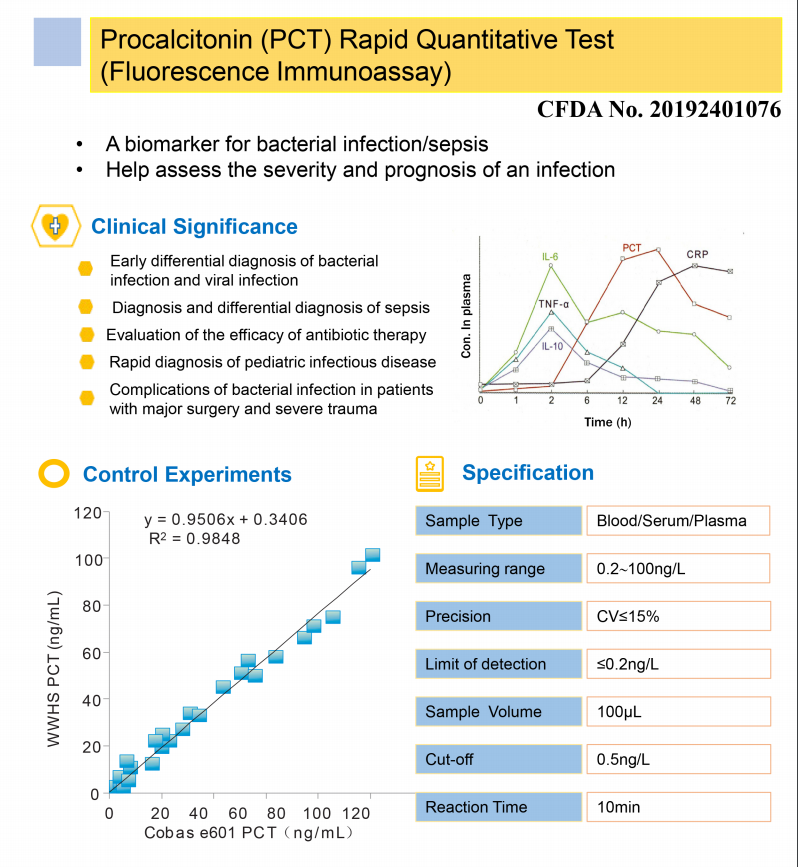
The product is used to determine the content of procalcitonin (PCT)
in whole blood, plasma and serum of human body and is mainly used
clinically for auxiliary diagnosis of bacterial infectious
diseases.
[Applicable Instrument]
NIR-1000 dry fluoroimmunoassay analyser produced by WWHS Biotech.
Inc.
【Precautions】
- Test card is single-use and it cannot be reused.
- Please inspect packaging integrity and validity of kit before use
and then unpack the product. If the product is stored at low
temperature, restore to room temperature (15℃-30℃) before unpacking
and use. Reagent cannot be used if packaging is damaged and the
validity period expires.
- Take the test card out of the aluminum foil bag and carry out
experiment in 15min. Do not place it in the air for a long time to
avoid dampness.
- It is required to strictly comply with the requirements for sample
collection and storage. If the sample is turbid, please centrifuge
and precipitate it before use.
- The kit contains products from animals. Eligible information about
animal source and sanitary condition cannot absolutely ensure
inexistence of infectious pathogen. Therefore, these products
should be disposed of as latent infective material, and all
samples, reagents and latent contaminants should be disinfected and
disposed of according to relevant local regulations.
- Too high or too low hematocrit of red cells may affect whole blood
test result, so verification should be conducted using other
methods .