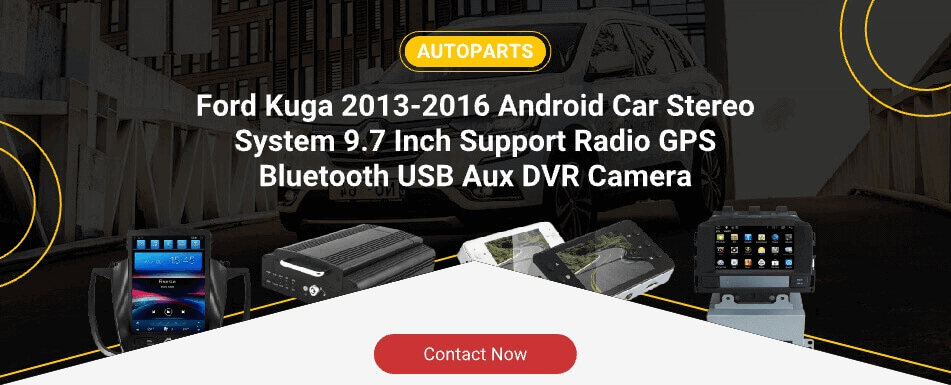
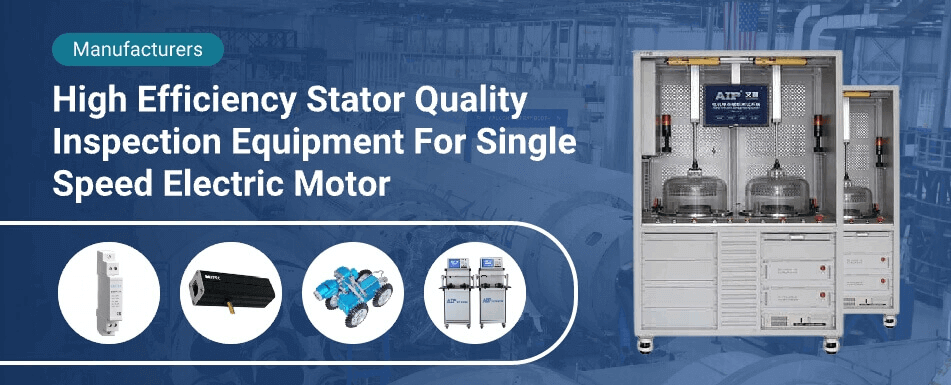
Popular Products
portable wine cooler bag
3d birthday card
carabiner multi tool
microscope slide box
worker boot
super glue gun
polka dot grosgrain ribbon wholesale
knife sharpening
single pole motor
music greeting cards free
从全球供应商中脱颖而出?
获得大量高质量一对一询盘? 外贸旺站:建立多语言网站 贸易通:全球本地化推广
获得大量高质量一对一询盘? 外贸旺站:建立多语言网站 贸易通:全球本地化推广
了解更多详情,请致电:
400-6516918 在线申请
Refine Search
insect bite
ALFA ROMEO 60562537
buy bmw
photo black white
banding machine steel
rear bicycle rack
t5 led tube diameter
aquarium fish light
surgical scrub brush
energy drink production
China Feature Products
Total 51,970,549
New Products
New Companys
Videos
Don't miss out on FREE new product updates!
Subscribe
Friendly Links:
Featured Company
Weltech Group Co., Limited
Yongkang Aoyue Tools Co.,Ltd
Optical instrument branch of Qingdao petro gulf equipment co.,ltd
Hileading Long International LTD
Ningbo Nijia Electronics Technology Co.,Ltd
Shenzhen Lian Da Technology Industrial Co.,Ltd
GLANWIN (Products) CO., LTD.
Shenzhen Xuancai Electronic Co., Ltd
Yunhe Heyi Wooden Toys Co., Ltd.
SHENZHEN JIATUO PLASTIC MACHINERY CO.,LTD
MIANYANG XIANFENG MEDECAL INSTRUMENT CO.,LTD
Guangzhou Ruisu Intelligent Technology Co., Ltd.
Weltech Group Co., Limited
Yongkang Aoyue Tools Co.,Ltd
Optical instrument branch of Qingdao petro gulf equipment co.,ltd
Hileading Long International LTD
Ningbo Nijia Electronics Technology Co.,Ltd
Shenzhen Lian Da Technology Industrial Co.,Ltd
GLANWIN (Products) CO., LTD.
Shenzhen Xuancai Electronic Co., Ltd
Yunhe Heyi Wooden Toys Co., Ltd.
SHENZHEN JIATUO PLASTIC MACHINERY CO.,LTD
MIANYANG XIANFENG MEDECAL INSTRUMENT CO.,LTD
Guangzhou Ruisu Intelligent Technology Co., Ltd.
Weltech Group Co., Limited
Yongkang Aoyue Tools Co.,Ltd
Optical instrument branch of Qingdao petro gulf equipment co.,ltd
Hileading Long International LTD
Ningbo Nijia Electronics Technology Co.,Ltd
Shenzhen Lian Da Technology Industrial Co.,Ltd