CITEST DIAGNOSTICS INC. |
|
Verified Suppliers
|
|
| Principle | Chromatographic Immunoassay |
| Format | Cassette |
| Specimen | Feces |
| Certificate | CE |
| Reading Time | 10 minutes |
| Pack | 10 T |
| Storage Temperature | 2-30°C |
| Shelf Life | 2 Years |
Vibrio cholerae O1/O139 Rapid Test (Feces) With CE
A rapid test for the qualitative detection of Vibrio cholerae O1 and Vibrio cholerae O139 in the specimens of human feces. For professional in vitro diagnostic use only.
Application:
The Vibrio cholerae O1/O139 Combo Rapid Test Cassette (Feces) is a rapid chromatographic immunoassay for the qualitative detection of Vibrio cholerae O1 and Vibrio cholerae O139 in human feces to aid in the diagnosis of Vibrio cholerae O1 or Vibrio cholerae O139 infection.
Description:
Cholerae is an acute watery diarrheal disease caused mainly by Vibrio choleraee serogroup O1 and less commonly by V. cholerae O139. cholerae can lead to severe diarrhea and death if untreated. V. cholerae O1 and V. cholerae O139 are transmitted through fecal-oral contamination, and cholera is thus predominantly associated with lack of safe drinking water, proper sanitation and personal hygiene. cholerae is an important public health problem in many parts of Asia, Africa and Latin America . Globally, 3–5 million cases and over 100,000 deaths occur annually due to cholera .
Countries facing complex emergencies are more vulnerable to cholera outbreaks . The etiological agent of cholera has been identified as Vibrio cholerae (V. cholerae), a gram negative bacterium, which is generally transmitted to humans via contaminated water and food. The species V. cholerae is divided into several serogroups on the basis of O antigens. The subgroups O1 and O139 are of special interest because both can cause epidemic and pandemic cholera.
It is critical to determine as quickly as possible the presence of V. cholerae O1 and O139 in clinical specimens, water, and food so that appropriate monitoring and effective preventive measures can be undertaken by public health authorities.
The Vibrio cholerae O1/O139 Combo Rapid Test Cassette (Feces) is a
rapid chromatographic immunoassay for the qualitative detection of
Vibrio cholerae O1 and Vibrio cholerae O139 in human feces,
providing results in 10 minutes. The test utilizes antibodies
specific for VC O1 and VC O139 antigens to selectively detect VC O1
antigens and VC O139 antigens in human feces.
How to use?
Allow the test cassette, specimen, buffer and/or controls to reach
room temperature (15-30°C) prior to testing.
For Fecal Specimens:
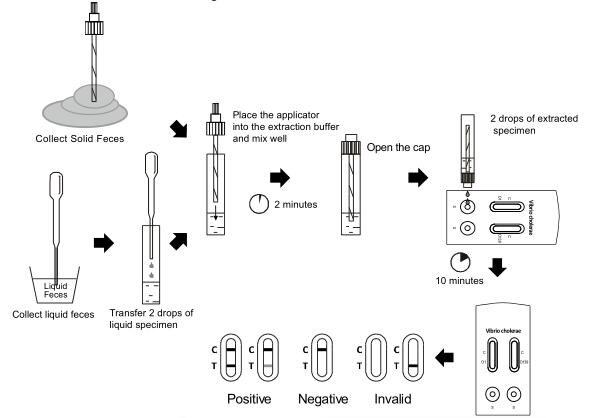
1. To collect fecal specimens:
Collect sufficient quantity of feces (1-2 ul or 1-2 g) in a clean,
dry specimen collection container to obtain maximum antigens (if
present). Best results will be obtained if the assay is performed
within 6 hours after collection. Specimen collected may be stored
for 3 days at 2-8°C if not tested within 6 hours.
For long term storage, specimens should be kept below -20°C.
2. To process fecal specimens:
For Solid Specimens:
Unscrew the cap of the specimen collection tube,then randomly stab
the specimen collection applicator into the fecal specimen in at
least 3 different sites to collect approximately 50 mg of
feces (equivalent to 1/4 of a pea). Do not scoop the fecal
specimen.
• For Liquid Specimens:
Hold the dropper vertically, aspirate fecal specimens, and then
transfer 2 drops of the liquid feces (approximately 80 μL) into the
specimen collection tube containing the extraction buffer.
Tighten the cap onto the specimen collection tube, and then shake
the specimen collection tube vigorously to mix the specimen and the
extraction buffer. Leave the tube alone for 2 minutes.
3. Bring the pouch to room temperature before opening it. Remove
the test cassette from the foil pouch and use it within one hour.
Best results will be obtained if the test is performed immediately
after opening the foil pouch.
4. Hold the specimen collection tube upright and open the cap onto
the specimen collection tube. Invert the specimen collection tube
and transfer 2 full drops of the extracted specimen (approximately
80μL) to each specimen well (S) of the test cassette, then start
the timer. Avoid trapping air bubbles in the specimen well (S). See
illustration below.
5. Read results at 10 minutes after dispensing the specimen. Do not read results after 20 minutes.
INTERPRETATION OF RESULTS
(Please refer to the illustration above)
VC O1 POSITIVE:* Two lines appear in VC O1 window. One colored line should be in
the control line region (C) and another apparent colored line
should be in the test line region (T).
*NOTE: The intensity of the color in the test line region (T) will vary
depending on the concentration of Vibrio cholerae O1 antigen
present in the specimen. Therefore, any shade of color in the test
line region (T) should be considered positive.
VC O139 POSITIVE:* Two lines appear in VC O139 window. One colored line should be in
the control line region (C) and another apparent colored line
should be in the test line region (T).
*NOTE: The intensity of the color in the test line region (T) will vary
depending on the concentration of Vibrio cholerae O139 antigen
present in the specimen. Therefore, any shade of color in the test
line region (T) should be considered positive.
NEGATIVE : One colored line appears in the control line region (C) . No line
appears in the test line region (T).
INVALID: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
Order Information
| Cat. No. | Product | Specimen | Pack |
| IVCA-602 | Vibrio cholerae O1 (VC O1) Rapid Test Cassette | Feces | 10T |
| IVCB-602 | Vibrio cholerae O139 (VC O139) Rapid Test Cassette | Feces | 10T |
| IVCAB-625 | Vibrio cholerae O1/O139 Combo Rapid Test Cassette | Feces | 10T |