JIAXING HENGJIE BIOPHARMACEUTICAL CO.,LTD. |
|
Verified Suppliers
|
Bovine Chondroitin Sulfate Sodium by GMP Manufacturer with DMF Documentation
Our company Jiaxing Hengjie Biopharmaceutical Co.,ltd. is able to produce and supply Bovine chondroitin sulfate that is GMP verified, and with DMF documentation.
Founded in 1997, our company is now a manufacturer of chondroitin sulfate for over 20 years. and Since 2012, Our compnay had been NSF-GMP verified manufacturer of chondroitin sulfate. And at the same year, we submit our DMF(Drug Master File) documentation in CTD Form of chondroitin sulfate to US FDA, and we get the DMF number : #26474. Also DMF Documentation is available for your review.
The Specification of bovine chondroitin sulfate sodium:
ITEMS | SPECIFICATIONS | TEST METHOD |
Appearance | White to off-white powder | Visual checking |
Identification | Infra Red Confirmed | (USP197K) |
| Sodium reaction | (USP191) |
Assay(ODB) | NLT90% | (CPC Titration) |
Loss on drying | Less than10% | (USP731) |
Protein | NMT6.0% | (USP41) |
Heavy Metal | NMT20PPM | (Method I USP231) |
PH (1%H2O solution) | 5.5-7.5 | (USP791) |
Specific Rotation | - 20°~ -30° | (USP781S) |
Residue on Ignition | 20%-30% | (dry base)(USP281) |
Organic Volatile Residual | NMT0.5% | (USP467) |
Clarity (5%H2O solution) | <0.35@420nm | USP41 |
Electrophoretic purity | NMT2.0% | (USP726) |
Total Bacteria Count | <1000CFU/g | (USP2021) |
Yeast & Mold | <100CFU/g | (USP2021) |
Salmonella | Negative | (USP2022) |
E.Coli | Negative | (USP2022) |
Staphylococcus Aureus | Negative | (USP2022) |
Particle size | 100% through 80 mesh | Pass |
What is the function of chondroitin sulfate in joints ?
Please see below picture
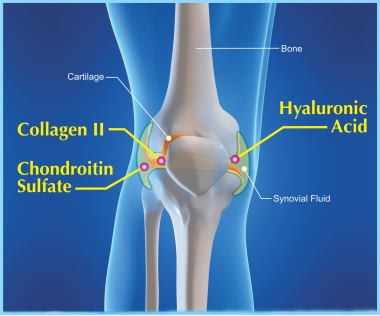
Studies show that bovine chondroitin sulfate sodium helps to slow the breakdown of cartilages in joints because it is considered as the building blocks for cartilage. It is widely used in dietary supplements products intended for osteoarthritis usually in combination with other joint health ingredients including D-glucosamine sulfate potassium chloride, D-glucosamine sulfate sodium chloride, D-glucosamine Hydrochloride, collagen, shark cartilage powder and Hyaluronic acid.
The Application of bovine Ch
Tablets | Bovine chondroitin sulfate sodium is widely used in combination with Glucosamine and MSM(Methyl-Sulfonyl-Methane) in Tablets as dietary supplements intended for joint health |
Capsules | Bovine chondroitin sulfate sodium could also be served in capsules form together with glucosamine and MSM. |
Powder form | Bovine chondroitin sulfate could also be produced into powder form in sachets |
Quality Management System
The packing of Bovine chondroitin sulfate sodium USP41 CPC 90%
